JKBOSE 10th Class Science Solutions Chapter 8 Carbon And Its Compounds
JKBOSE 10th Class Science Solutions Chapter 8 Carbon And Its Compounds
JKBOSE 10th Class Science Solutions Chapter 8 Carbon And Its Compounds
Jammu & Kashmir State Board JKBOSE 10th Class Science Solutions
J&K class 10th Science Carbon And Its Compounds Textbook Questions and Answers
BASIS AND BASICS
◆ Carbon is a versatile element that forms the basis for all living organisms and many things which we use in our daily life.
◆ Chemical bond is the attractive forces which hold the atoms or ions together in a chemical species.
◆ Causes of chemical bonding (i) the tendency of the atoms of the various elements to acquire the stable nearest noble gas configuration i.e., to complete their octet and (ii) to acquire a state of minimum energy.
◆ During the formation of a chemical bond, energy is given out.
◆ Noble gases are chemically less reactive and have eight electrons in their valence shells (except helium).
◆ Octet rule is the tendency of an atom of the element to have eight electrons in its valence shell.
◆ Molecule is an electrically neutral cluster of mutually bonded atoms. e.g. CH4, NH3, H2O etc.
◆ Chemical bonds are mainly of three types (a) Ionic or electrovalent bond (b) Covalent bond (c) Co-ordinate bond. “
◆ Covalent bond. It is the bond formed by equal contribution and mutual sharing of electrons between two atoms so that both the atoms get their octet (or duplet) complete.
◆ Covalent compounds. The compounds containing covalent bonds are called covalent compounds.
◆ Covalency. It is the number of electrons contributed by an atom of the element for mutual sharing during the formation of a covalent bond.
◆ Bond pair of electrons is the pair of electrons shared between two atoms.
◆ Lone pair of electrons is the electron pair present on one atom which does not take part in sharing.
◆ Single, double and triple bonds are formed by the mutual sharing of one, two and three electron pairs between two atoms.
◆ Organic Compounds. These are made of C, H, O, N, S, P, halogens and have covalent bonds. Or The organic compounds include hydrocarbons and their derivatives.
◆ Catenation. It is the property by virtue of which atoms of the same element get linked together through covalent bonds so as to form long straight, branched or closed chains or rings. For example, carbon atom shows catenation to maximum extent.
◆ Tetracovalency or Tetravalency. Carbon atoms shows tetracovalency because it has four electrons in its valence shell.
◆ This large variety of compounds is formed by carbon because of its tetravalency and the property of catenation.
◆ Structural formula. It gives us t the relative arrangements of bonded atoms in a molecule.
◆ Functional group. An atom o or group of atoms or some other characteristics structural feature which when present, in a molecule gives some special properties to a compound e.g., OH alcoholic group, – CHO aldehydic group. J
◆ Homologous series. It is a series of compounds having similar structural formulae, same functional group and hence, similar chemical properties.
◆ IUPAC System stands for International Union of Pure and Applied Chemistry System.
◆ Saturated hydrocarbons are those compounds which contain C – C single bonds in their molecules.
◆ Unsaturated hydrocarbons. They include alkenes and alkynes. These are those compounds which contain C = C bonds or C ≡ C bonds in their molecules.
◆ Pyrolysis. It is decomposition of higher molecular weight hydrocarbons into lower molecular weight hydrocarbons on heating to a high temperature in the absence of air.
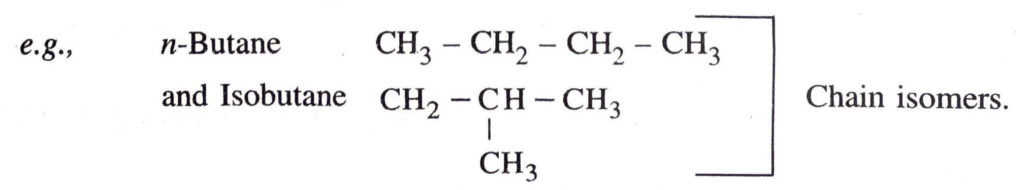
◆ Isomerism. The compounds having same molecular formula but different properties are called isomers and this phenomenon is called isomerism.
◆ Carbon and its compounds are some of our major source of fuels.
◆ Ethanol and ethanoic acid are carbon compounds of importance in our daily lives.
◆ The action of soaps and detergents is based on the presence of both hydrophobic and hydrophilic groups in the molecule and this helps to emulsify the oily dirt and hence remove it from clothes.
IMPORTANT TERMS AND FACTS TO MEMORISE
⇒ Hydrocarbons. These are the covalent compounds of carbon and hydrogen.
⇒ Organic Chemistry. It is the study of hydrocarbons and their derivatives.
⇒ Allotropy. It is the phenomenon of existence of an element in different forms having different physical properties but same or slightly different chemical properties.
⇒ Alkanes. These are the hydrocarbons containing C- C and C H single bonds. These are also called saturated hydrocarbons. General formula of alkanes is CnH2n + 2 n = : 1, 2, 3………
⇒ Unsaturated Hydrocarbons. These are the hydrocarbons containing C = C or – C ≡ C- bonds in their molecules.
TEXT BOOK QUESTIONS (SOLVED)
Q. 1. What would be the electron dot structure of carbon dioxide which has the formula CO₂ ?
Ans. :Ö::C::Ö:
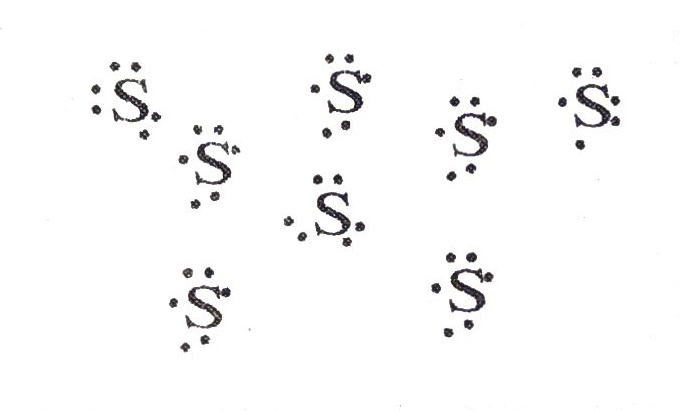
Q. 2. What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur ? (Hint: The eight atoms are joined together in the form of a ring).
Ans.
Q. 3. How many structural isomers can you draw for pentane ?
Ans. Pentane has three structural isomers.
These are
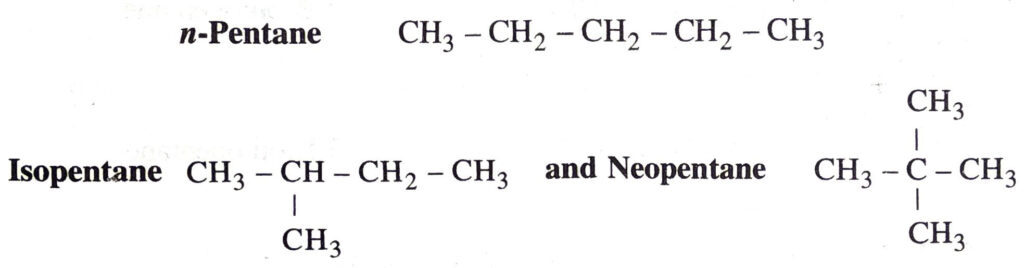
Q. 4. What are the two properties of carbon which lead to the large number of carbon compounds we see around us ?
Ans. These are 1. Tetracovalency 2. Catenation.
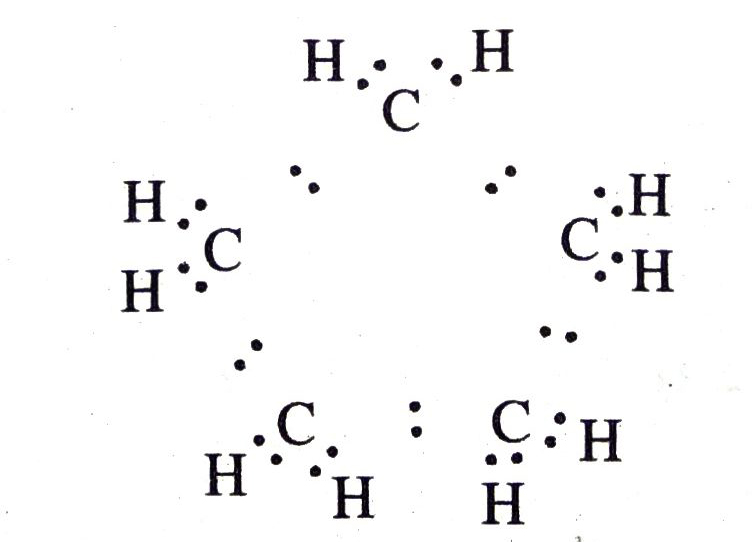
Q. 5. What will be the formula and electron dot structure of cyclopentane ?
Ans. Formula of cyclopentane is C5H10 and its electron dot structure is :
Q. 6. Draw the structures of the following compounds :
(a) Ethanoic acid
(b) Butanone
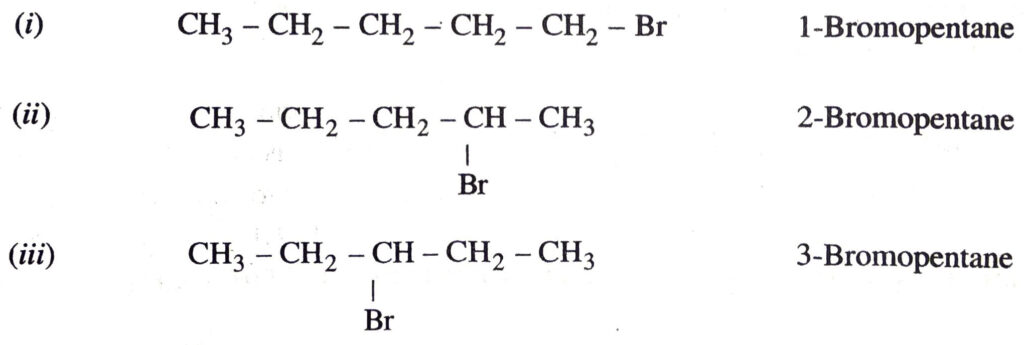
(c) Bromopentane
(d) Hexanal.
Are structural isomers possible for bromopentane ?
Ans. (a) Ethanoic acid has structure
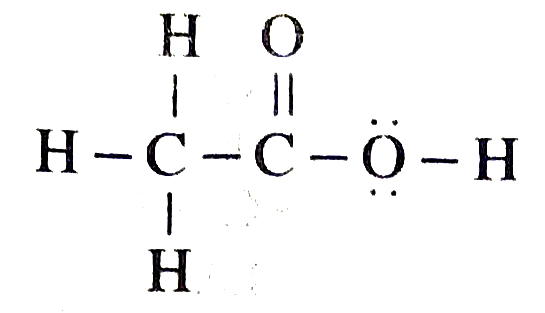
(b) Butanone
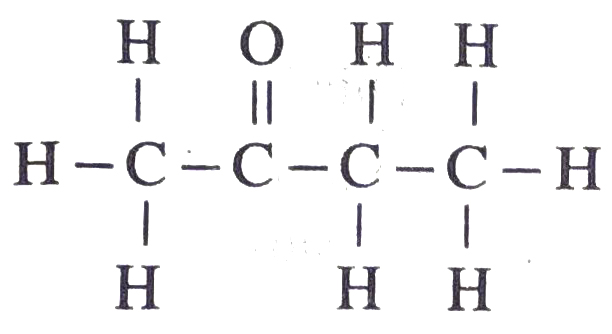
(c) Bromopentane. There are three structural isomers possible for bromopentane. These are
(d) Hexanal
CH3 – CH2 – CH2 – CH2 – CH2 – CHO –
Q. 7. How would you name the following compounds ?
(a) CH3 – CH2 – Br
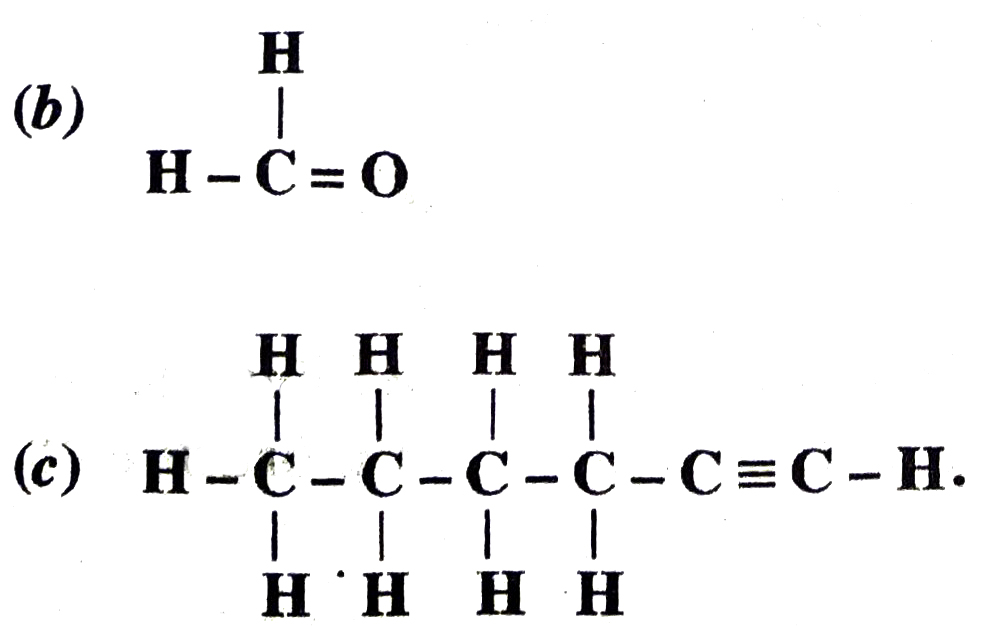
Ans. (a) Bromoethane
(b) Methanal Saistige
(c) Hex-1-yne.
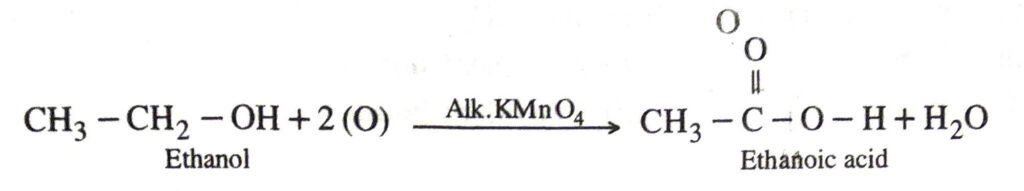
Q. 8. Why is the conversion of ethanol to ethanoic acid an oxidation reaction ?
Ans. This is because in this reaction oxygen gets added to ethanol.
Q. 9. A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used ?
Ans. A mixture of ethyne and air is not burnt for welding. This is because air also contains nitrogen along with oxygen. Nitrogen will also burn in oxygen producing oxides of nitrogen such as nitrous oxide (NO) and nitrogen dioxide (NO₂) which cause pollution.
Q. 10. How would you distinguish experimentally between an alcohol and a carboxylic acid ?
Ans. The following two tests are used :
1. Litmus test. Treat the given compound with blue litmus solutions. If the blue litmus solution turns red, it is a carboxylic acid and if does not turn red, it is an alcohol.
2. Sodium bicarbonate test. Add some sodium bicarbonate solution to the given compound. If their is a brick evolution of a colourless and odourless gas (CO₂) which turns freshly prepared lime water milky, it is carboxylic acid and if their is no effervescence, it is an alcohol.
Q. 11. What are oxidising agents ?
Ans. The substances which can oxidise other substances by giving oxygen are called oxidising agents.
Examples. Alkaline potassium permanganate solution, acidified potassium dichromate solution etc.
Q. 12. Would you be able to check if water is hard using a detergent ?
Ans. No, we can’t check whether the water is hard or soft using a detergent.
Q. 13. People use a variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat it with a paddle, scrub with a brush or the mixture is agitated necessary to get clean clothes.
Ans. This is because when soap molecules dissolve in dirt, the dirt is somewhat loosened from the clothes and in order to remove it from clothes, the clothes have to be beaten on a stone or beaten with a paddle or scrubbed with a brush or mixture has to be agitated in washing machines.
TBE TEXT BOOK EXERCISES (SOLVED)
Q. 1. Ethane, with the molecular formula C2H6 has :
(a) 6 covalent bonds
(b) 7 covalent bonds
(c) 8 covalent bonds
(d) 9 covalent bonds.
Ans. (b).
Q. 2. Butanone is a four-carbon compound with the functional group :
(a) carboxylic acid
(b) aldehyde
(c) ketone
(d) alcohol.
Ans. (c).
Q. 3. While cooking, if the bottom of the vessel is getting blackened on the outside, it means that :
(a) the food is not cooked completely
(b) the fuel is not burning completely
(c) the fuel is wet
(d) the fuel is burning completely.
Ans. (b).
Q. 4. Explain the nature of the covalent bond using the bond formation in CH3Cl.
Ans. The formation of CH3Cl can be represented as :
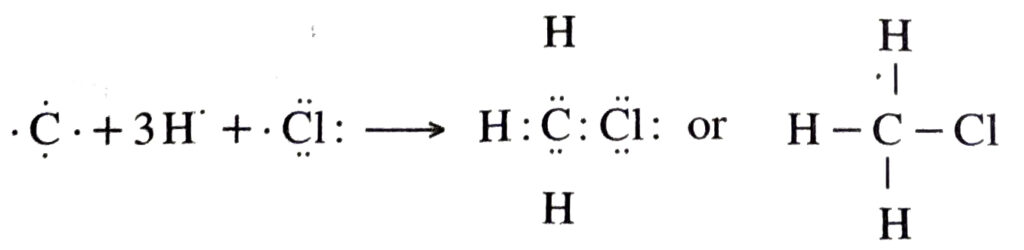
Carbon forms single covalent bonds with three H-atoms and one Cl-atom by sharing one electron pair with each C-H bonds are non-polar. But C-Cl bond is polar this is because C and H leave almost same electronegativity whereas Cl has more electronegativity than carbon.
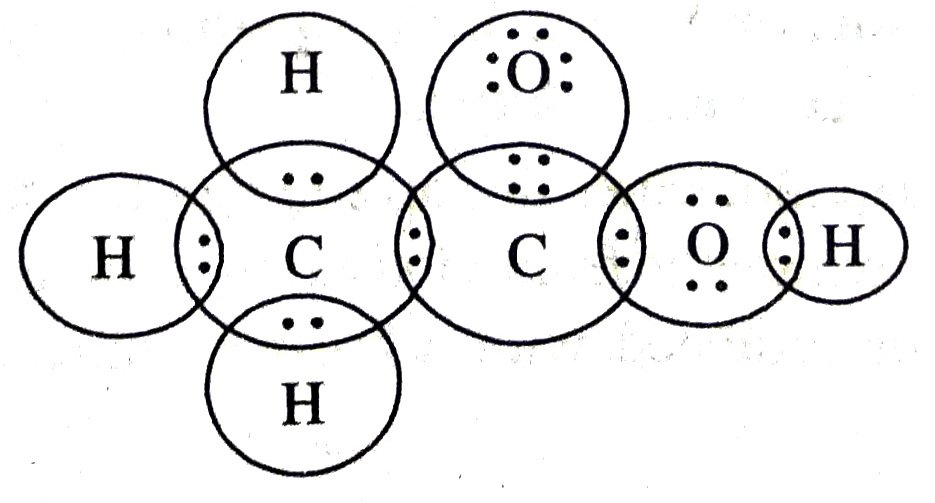
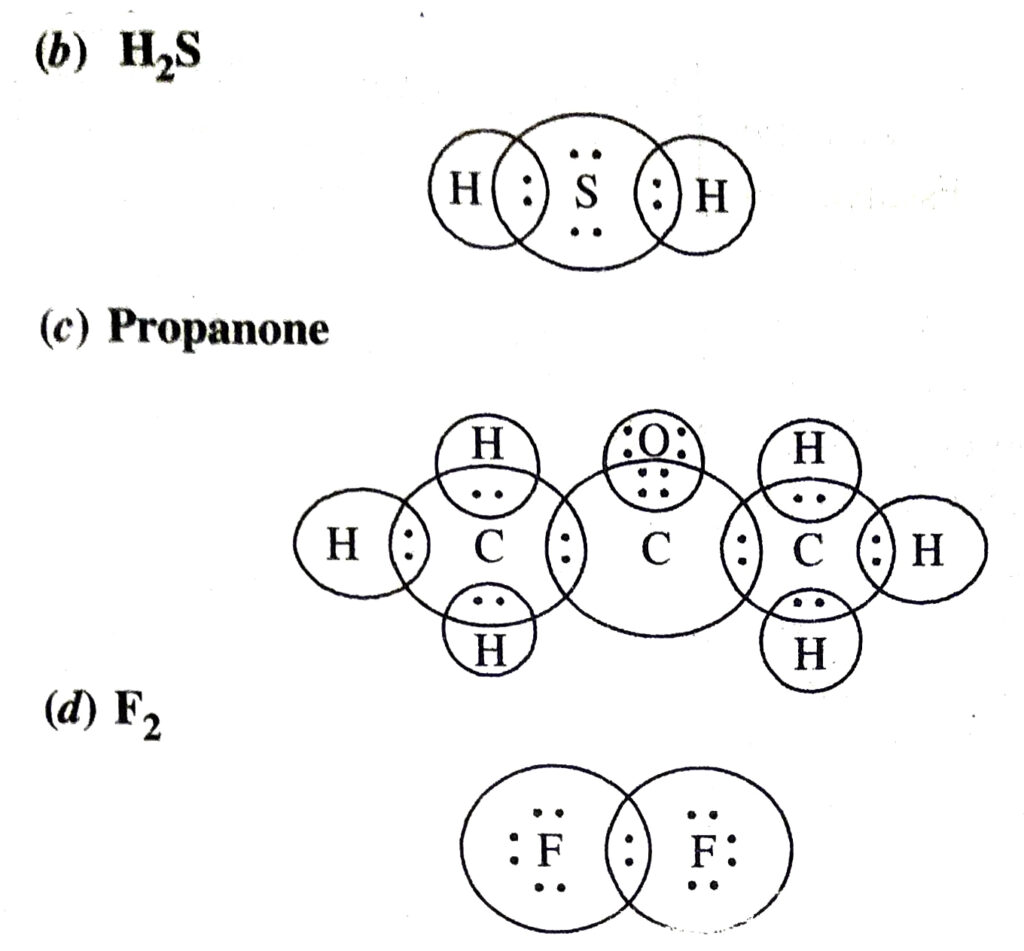
Q. 5. Draw the electron dot structures for :
(a) ethanoic acid
(b) H₂S
(c) propanone
(d) F₂.
Ans. (a) Ethanoic acid
Q. 6. What is an homologous series ? Explain with an example.
Ans. A series of compounds having similar structural formulae, same functional group and hence similar chemical properties is called a homologous series. In the homologous series any two adjacent members differ by CH₂ unit in their molecular formulae.
For example homologous series of aldehydes (or alkanals) can be represented as:
H – CHO Methanal
CH3 – CHO Ethanal
CH3 – CH2 – CHO Propanal
CH3 – CH2 – CH2 – CHO Butanal
CH3 – CH2 CH2 – CH2 – CHO Pentanal and so on.
Q. 7. How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties ?
Ans. Distinction between ethanol and ethanoic acid :

Q. 8. Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also ?
Ans. Soap molecule has two ends, one is hydrophillic, and it dissolves in water, while the other end is hydrophobic, and it dissolves in hydrocarbons. When soap is at the surface of water, the hydrophobic tail of soap will not be soluble in water and the soap will align along the surface of water with the ionic end in water and the hydrocarbon ‘tail’ pointing out of water. Inside water, these molecules have a unique orientation which keeps the hydrocarbon portion out of the water. This is achieved due to the formation clusters of molecules in which the hydrophobic tails are in the interior of the cluster and the ionic ends are on the surface of the cluster. This formation is called a micelle.
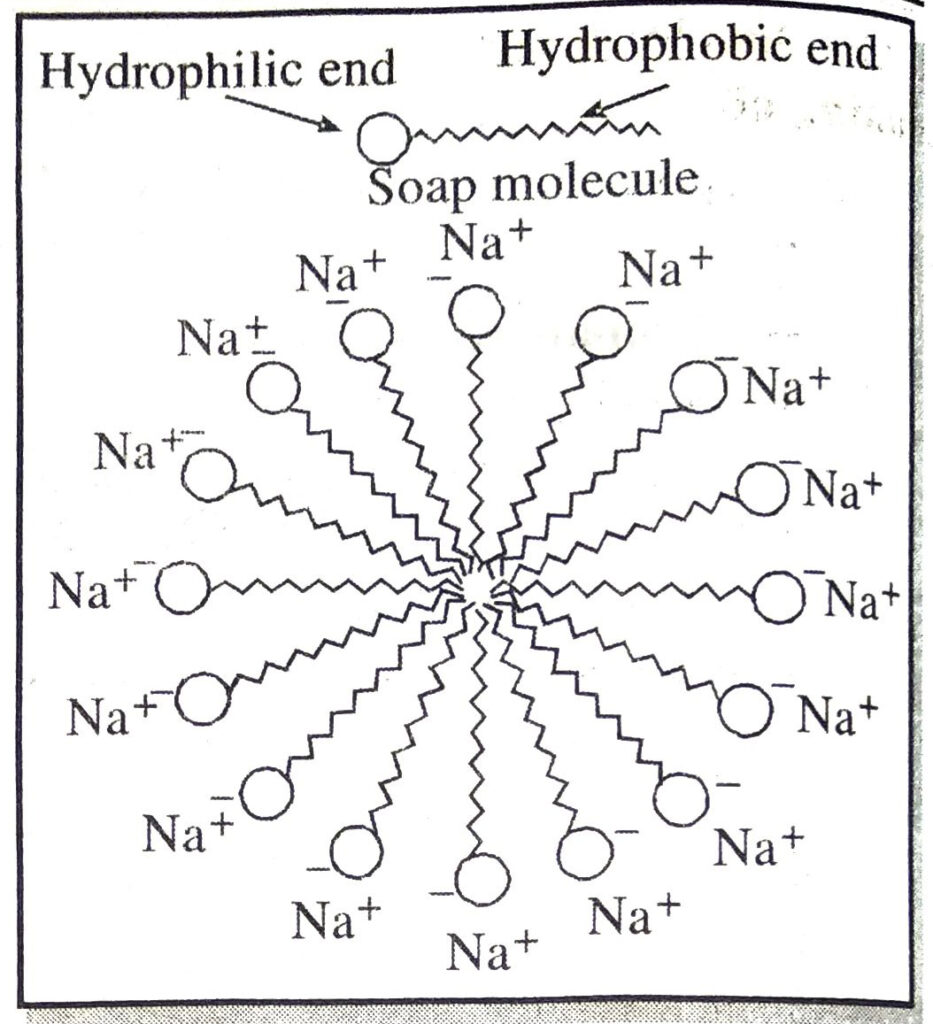
Q. 9. Why are carbon and its compounds used as fuels for most applications ?
Ans. Carbon and its compounds are used as fuels for most applications because they burn producing a large amount of heat and light.
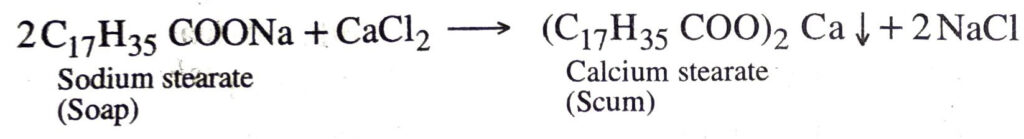
Q. 10. Explain the formation of scum when hard water is treated with soap.
Ans. When soap is added to hard water, the soluble calcium and magnesium salts present in it react with soap to give insoluble calcium salt of soap which produces scum.
Q. 11. What change will you observe if you test soap with litmus paper (red and blue) ?
Ans. Soap solution will turn red litmus paper blue because soap is alkaline in nature.
Q. 12. What is hydrogenation? What is its industrial application ?
Ans. The addition of hydrogen to unsaturated hydrocarbons in the presence of catalysts like palladium, platinum, nickel etc. to give saturated hydrocarbons is called hydrogenation.
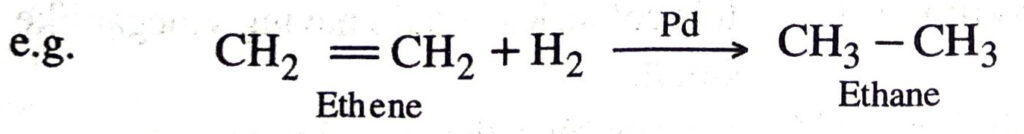
This reaction is used for hydrogenation of liquid vegetable oils using a nickel catalyst to get artificial or vanaspati ghee.
Q. 13. Which of the following hydrocarbons undergo addition reaction : C2H6, C3H8, C3H6, C2H2 and CH4 ?
Ans. Out of C2H6, C3H8, C3H6, C2H2 and CH4; C3H6 and C2H2 undergo addition reactions because they contain multiple bonds.
Q. 14. Give a test that can be used to differentiate chemically between butter and cooking oil.
Ans. Distinction between Butter and Cooking oil :

Q. 15. Explain the mechanism of the cleansing action of soaps.
Or
What is soap? Name one soap. Explain the cleansing action of soap. Draw diagram to illustrate answer.
Or
During washing of clothes, why people use different methods like beating the clothes on a stone, beating with a paddle or scurb with a brush or rock ‘n’ roll in washing machines? Is agitation of mixture of soap and water necessary for cleaning of clothes ?
Or
Why does micelle formation takes place when soap is added to water ?
Ans. Mechanism of cleaning action of Soap
Soap are sodium or potassium salts of higher fatty acids e.g. sodium palmitate, C15H31COO–Na+, sodium stearate, C17H35COO–Na+ etc. A molecule of soap consists of two parts:
(a) a long chain hydrocarbon part (C15H31 –, C17H35 …. etc.) which is soluble in oil and (b) ionic part on polar group, – COO–Na+ which is soluble in water. Thus a molecule of soap can be represented as :
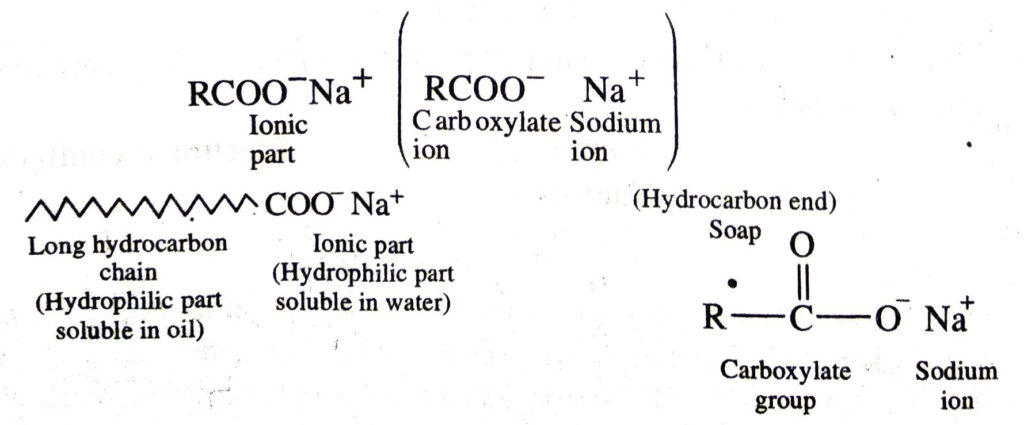
The long hydrocarbon chain is insoluble in water but soluble in oil and greases whereas the ionic or polar part is soluble in water. The dirty clothes contain greasy and oily substance (dirt). Soap molecules dissociate in water to g give carboxylate ion (RCOO–) and cation (Na+). When soap is added to dirty clothes dipped in water, the hydrocarbon part of carboxylate group dissolving in greasy or oily dirt particles where the polar (COO–) group remain attached to water. In this way each oil droplet acquires negative charge. These negative charged oil droplets called micelles can not associate and hence form a stable emulsion water. These small droplets along with dirt can be easily washed away with water by agitating the mixture of soap and water. Thus the soap helps in removing greasy dirt by producing a stable oil in water type emulsion. Also the soap reduces surface tension of water. Hence cloth is wetted more effectively and is cleaned. Agitation of mixture of soap and water is necessary to remove small droplets or micelles.
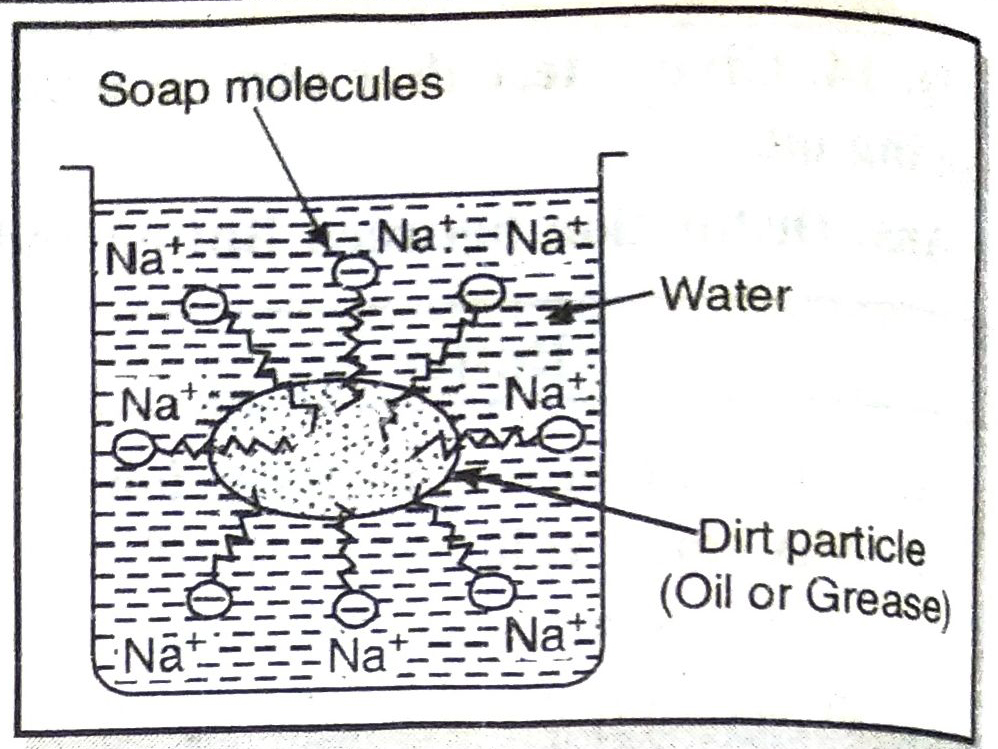
ADDITIONAL IMPORTANT QUESTIONS
LONG ANSWER TYPE QUESTIONS
Q. 1. (a) Define covalent bond. Explain with the help of examples.
Or
Define covalent bond. Draw the electronic structure of hydrogen, oxygen and nitrogen and state nature of bond formed in each case.
(b) What do you mean by multiple covalent bonding ? Explain with the help of examples.
Or
Draw electron dot structures of the following:
(i) H2 (ii) O2 (iii) N2 (iv) CH4 (v) C2H2 (vi) CO2.
Ans. (a) Covalent bond. The bond formed by equal contribution and mutual sharing of electrons between two atoms (same or different) so that both the atoms acquire the stable nearest noble gas configuration i.e. get their octet complete is called covalent bond.
The mutually shared electrons become the common property of both the bonded atoms.
The number of electrons contributed by an atom of the element for mutual sharing during the formation of a covalent bond is called its covalency.
Each pair of shared electrons is represented by putting a single line (—) between two atoms.
In the example given below :

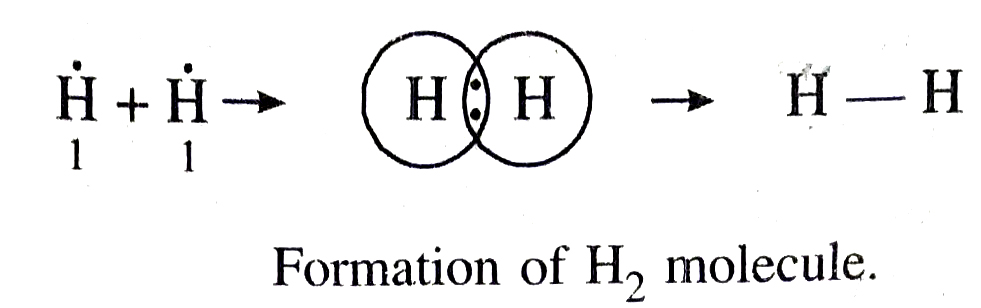
Examples. (i) Formation of a hydrogen molecule (H₂). At. no. of hydrogen = 1. It has one electron in the first orbit. When two hydrogen atoms approach each other they share their single electron present in their first orbits. Each hydrogen atom can now be thought of as having noble gas configuration of helium. It may be represented as:
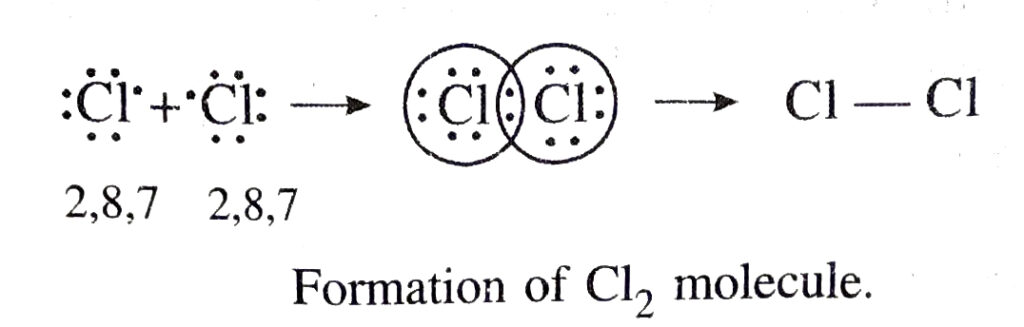
(ii) Formation of chlorine molecule. In this case, both the chlorine atoms have seven electrons in their outermost shell and they contribute one electron for mutual each to form a covalent bond. Thus, both the chlorine atoms acquire noble gas configuration of argon. This may be depicted as :
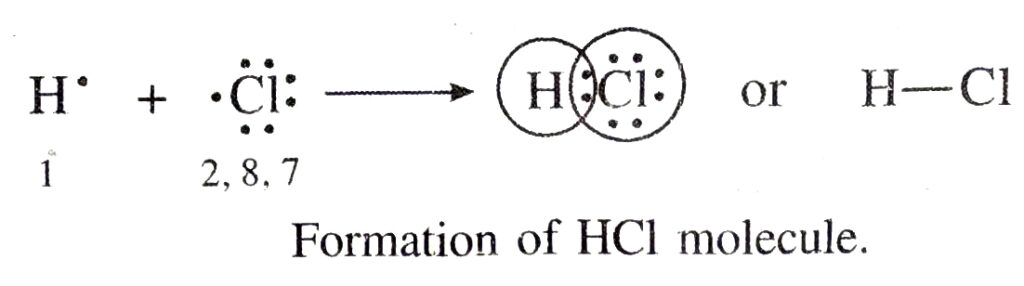
(iii) Formation of HCI molecule. Hydrogen atom has only one electron and chlorine atom has seven electrons in its valence shell. Therefore, by mutual sharing of electron pair between a hydrogen and a chlorine atom both the atoms acquire nearest noble gas configuration. Hydrogen atom acquires electronic configuration of helium where as chlorine atom gets electroric configuration of argon.
(b) Multiple covalent Bonding. If the two atoms share one electron pair, bond is known as single covalent bond and is represented by one dash (—). If the two atoms share two electron pairs, bond is known as double covalent bond and is represented by two dashes (=). If the two atoms share three electron pairs, bond is known as triple covalent bond and is represented by three dashes (≡).
In the case of H₂, Cl₂ and HCI molecules (given above) there are single bonds between the atoms.
Formation of Double Bond. Two oxygen atoms combine to form an oxygen molecule by sharing two electron pairs. In the formation of oxygen molecule, each oxygen atom has six electrons in the valence shell and require two electrons to complete the octet. Therefore, both the atoms contribute two electron pairs are shared and hence there is a double bond between the oxygen atoms. The covalency of oxygen is two.
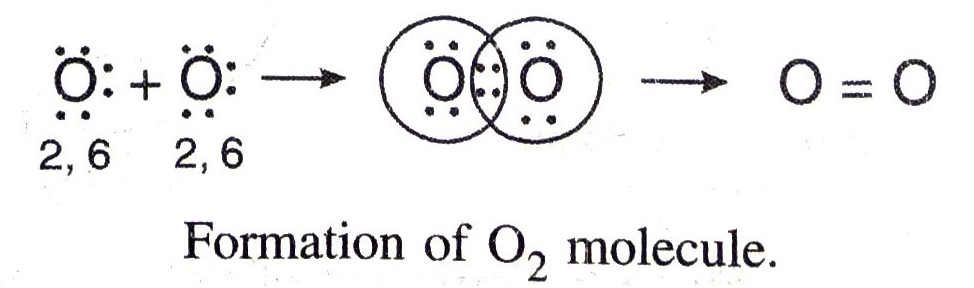
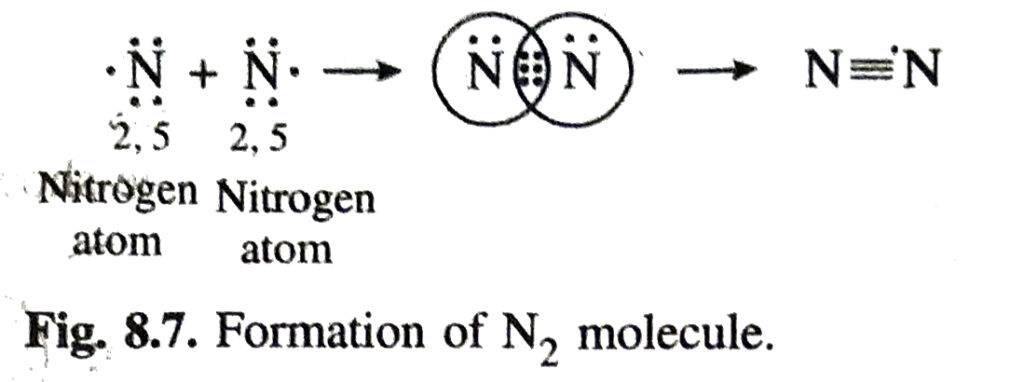
Formation of Triple Bond. In the formation of a nitrogen molecule, each of the bond nitrogen atoms having five electrons, provides three electrons to form three electrons pairs for sharing. Thus, a triple bond is formed between the two atoms. Here, covalency of nitrogen is three.
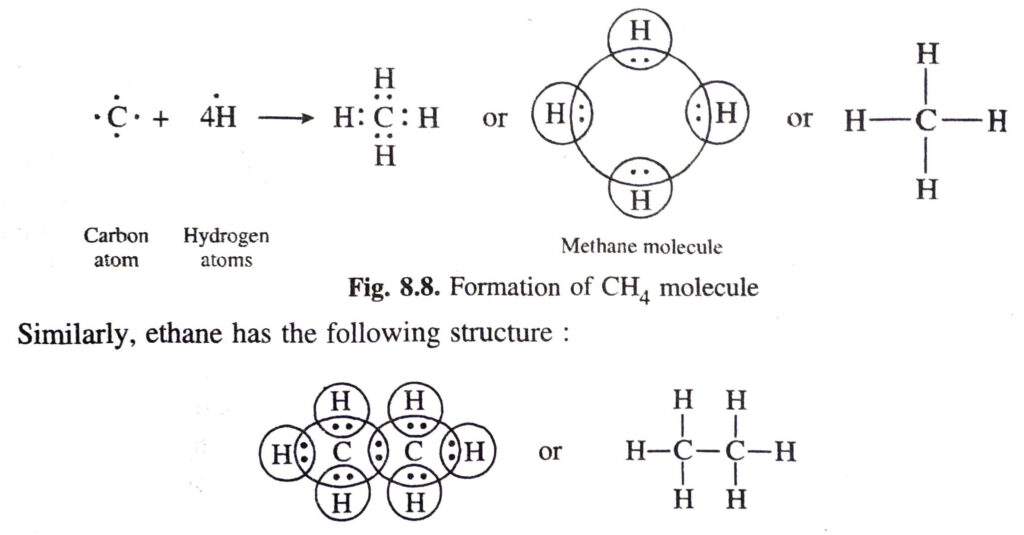
Q. 2. Discuss the formation of covalent bonds in molecules of (a) Methane (b) Carbon F tetrachloride (c) Water (d) Ammonia (e) Ethene (f) Carbon dioxide.
Ans. (a) Covalent bonds in methane (CH4) molecule. The atomic number of carbon is 6. Its electronic configuration is 2, 4. This means that carbon atom has four valence electrons. These are shared by the electrons of four hydrogen atoms. As a result, the carbon atom gets linked to four hydrogen atoms by four covalent bonds. The formation of methane molecule may be shown as follows:
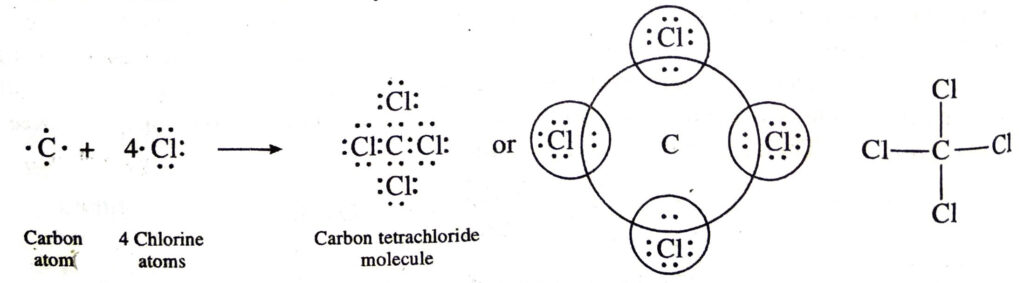
(b) Covalent bonds in carbon tetrachloride (CCl4) molecule. The atomic number of carbon is 6. Its electronic configuration is 2, 4. The four electrons present in the valence shell of carbon atom are shared by the unpaired electrons of four chlorine atoms (2, 8, 7). Thus, carbon atom gets linked to four chlorine atoms by four covalent bonds. The formation of carbon tetrachloride molecule may be shown as follows:
(c) Covalent bonds in water (H2O) molecule. The atomic number of oxygen is 8. Its electronic configuration is 2, 6. This means that oxygen atom has six valence electrons. In order to have eight electrons in its valence shell oxygen atom shares two electrons with the electrons of two hydrogen atoms. Thus, oxygen atom gets linked to hydrogen atoms by two covalent bonds. The formation of water molecule may be shown as below:
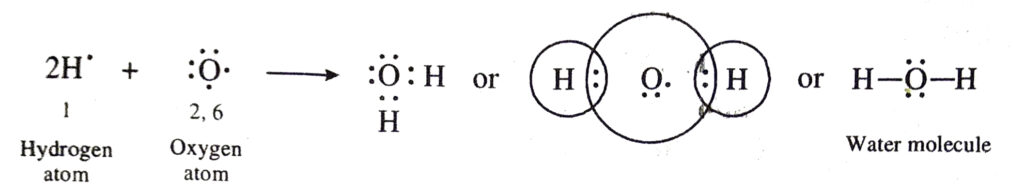
(d) Covalent bonds in ammonia (NH3) molecule. The atomic number of nitrogen is 7. Its electronic configuration is 2, 5. Nitrogen atom has five valence electrons. In order to have eight electrons in the valence shell, the nitrogen atom shares three electrons with the electrons of three hydrogen atoms. Thus, nitrogen atom gets linked to three hydrogen atoms by three covalent bonds. The formation of ammonia molecule may be shown as follows:
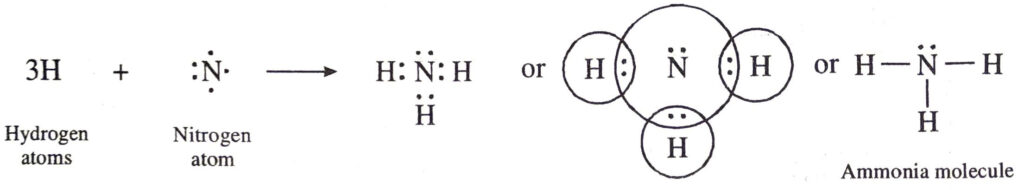
(e) Covalent bonds in ethylene (C2H4) molecule. Ethylene molecule has two carbon atoms. Each carbon atom shares two electrons with the two hydrogen atoms. At the same time, both the carbon atoms mutually share two electrons each. Thus both the carbon atoms get linked by double bond. Each carbon atom also gets linked to two hydrogen atoms by single bonds. The formation of ethylene molecule may be shown as follows:
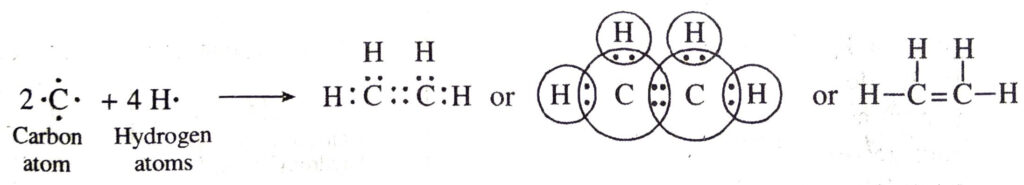
(f) Covalent bonds in carbon dioxide (CO2) molecule. Carbon atom has four electrons. Each oxygen atom has six valence electrons (2, 6). The carbon atom shares its electrons with the electrons of the two oxygen atoms. As a result, the carbon atoms get linked to the oxygen atoms by double bonds. The formation of carbon dioxide molecule may be shown as follows:
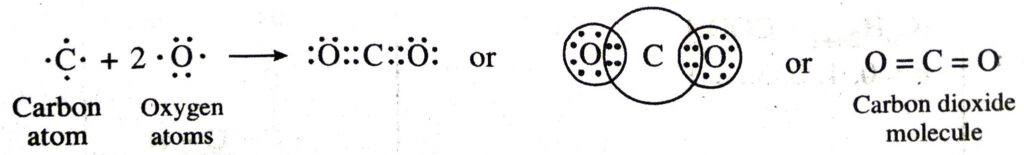
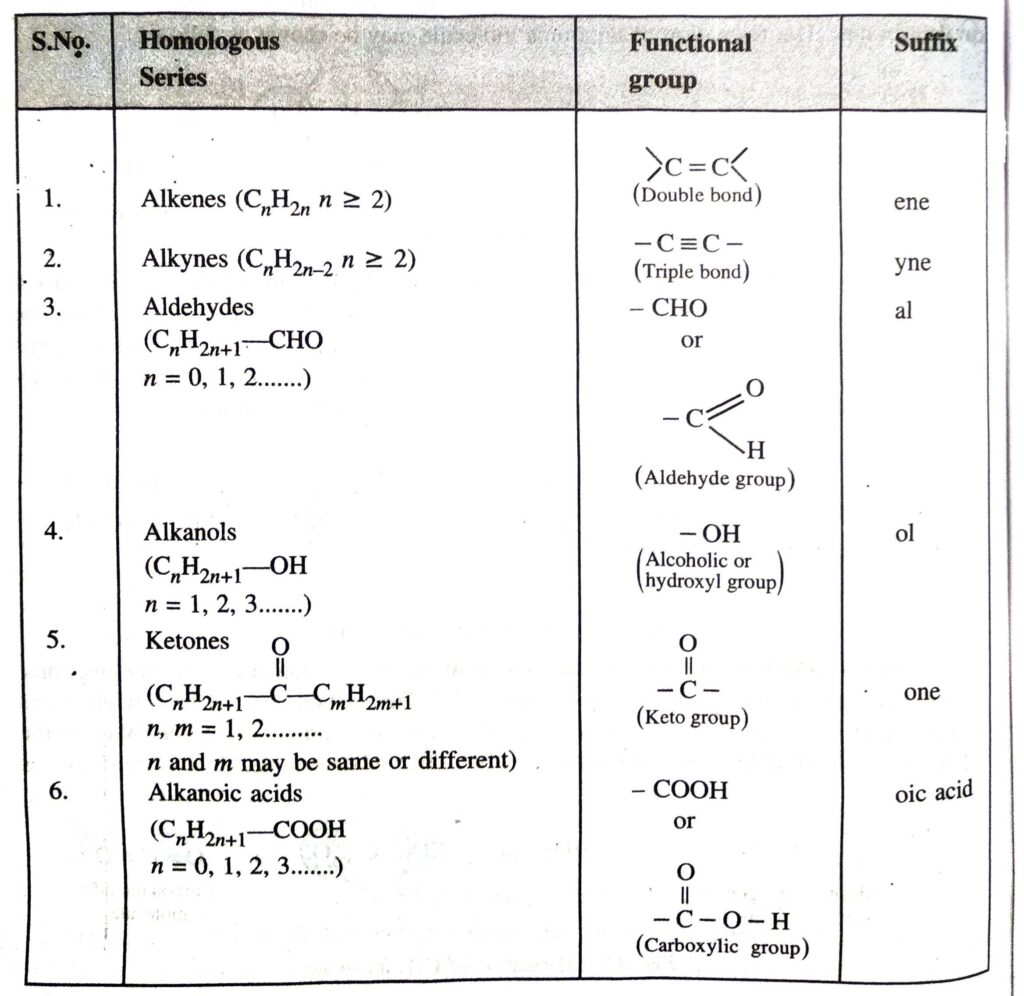
Q. 3. Give the functional groups and their suffixes present in :
1. Alkenes
2. Alkynes
3. Aldehydes
4. Alkanols
5. Ketones
6. Alkanoic acid
Or
Describe briefly the various homologous series of organic compounds.
Ans.
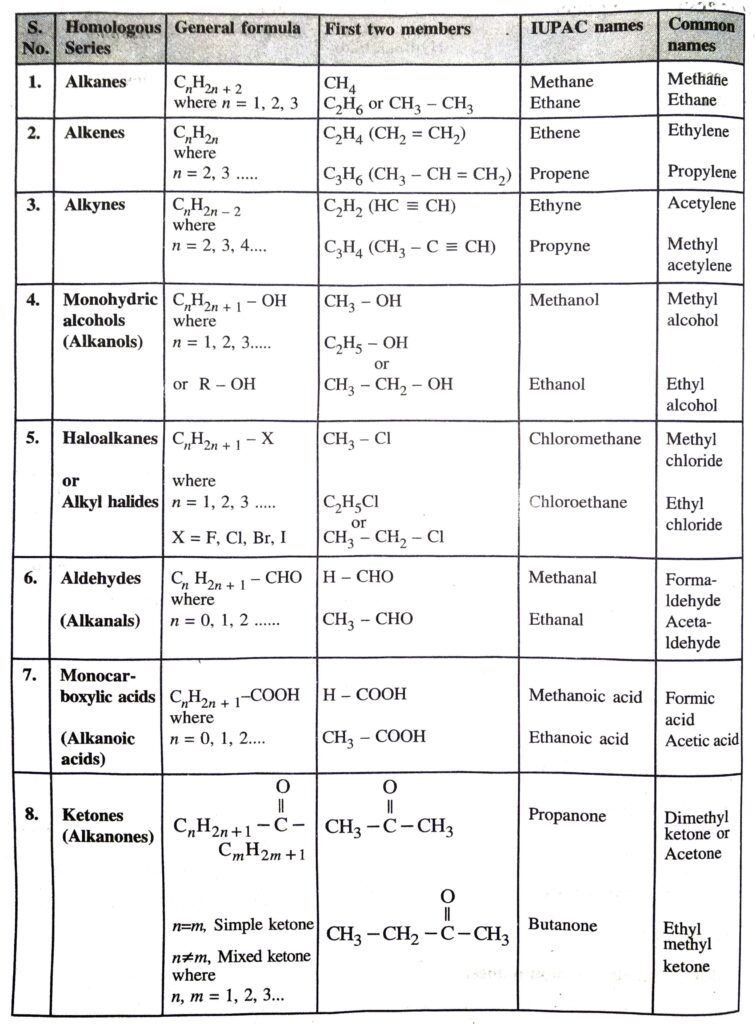
Q. 4. Give general formulae and IUPAC names of first two members of :
1. Alkanes
2. Alkenes
3. Alkynes
4. Monohydric alcohols
5. Haloalkanes
6. Aldehydes
7. Monocarboxylic acids
8. Alkanones
Also give their common names.
Ans.
Q. 5. Describe the important chemical properties of carbon compounds. Illustrate your answer with examples. Give equation of reactions in each case.
Or
Describe substitution and addition reactions as chemical properties of compounds.
Ans. Some important chemical properties of carbon compounds are :
1. Combustion. The carbon compounds on burning in a free supply of air or oxygen produce carbon dioxide, water and a large amount of heat and light energy.
Examples
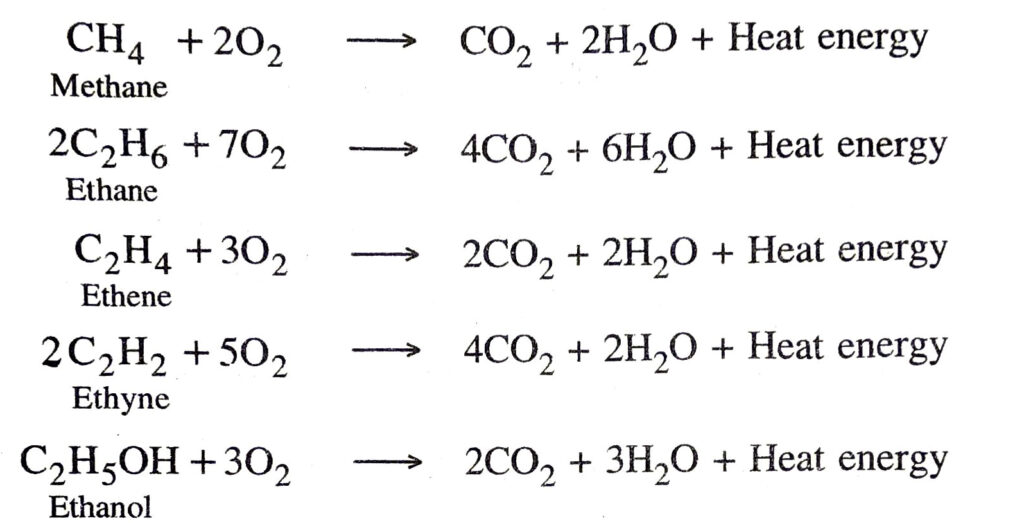
Saturated hydrocarbons generally burns with a clean flame whereas unsaturated hydrocarbons burn with a yellow flame and produce a large amount of black smoke. Also the saturated hydrocarbons also burn in the limited supply of air with a sooty flame. 2. Addition reactions. The chemical reactions in which two reactant molecules get added to form a single molecule are called addition reactions. During the addition reaction one of the reactant molecules must contain a multiple bond.
Unsaturated hydrocarbons (alkenes and alkynes) undergo addition reactions.
Some examples are
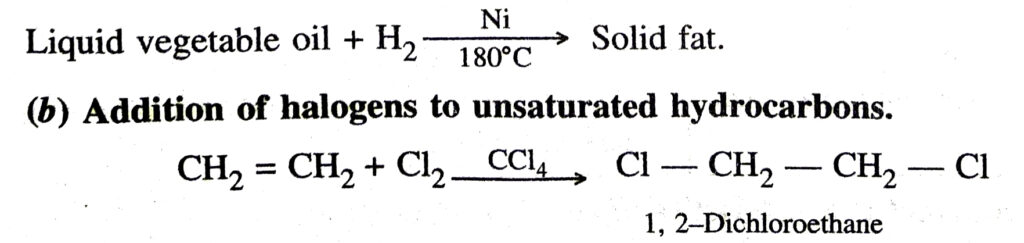
(a) Addition of hydrogen
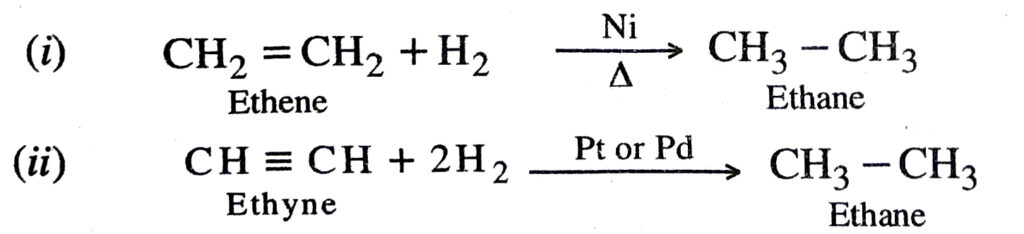
The above reaction is also called catalytic hydrogenation. This reaction is used for the hydrogenation of liquid vegetable oils from liquid vegetable oils (which are long unsaturated hydrocarbon chains)
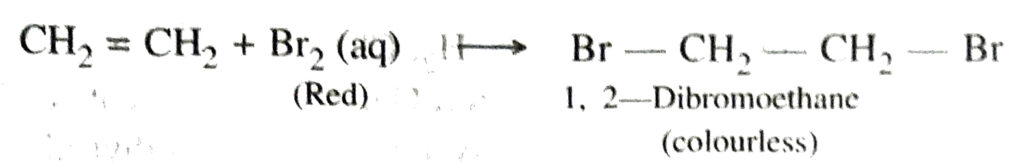
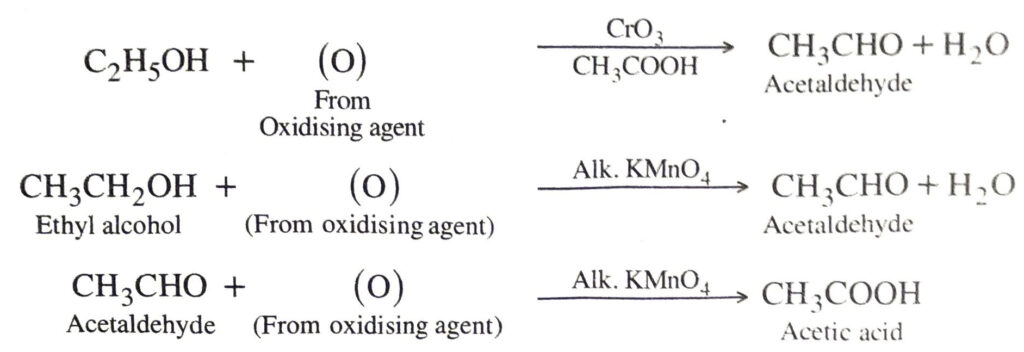
(c) Oxidation. In addition to combustion (which involves oxidation) carbon compounds also undergo oxidation reactions.
Examples. Alcohols are oxidised to aldehydes and ketones. The aldehydes and ketones are further oxidised to monocarboxylic acids.
(d) Substitution reactions. These reaction involve the replacement of an atom or group of atoms from a molecule by another atom or group without any change is structure in remaining part of the molecule.
Saturated hydrocarbons undergo substitution reactions.
Examples:

Another example is from allyl halide which also undergo substitution reactions
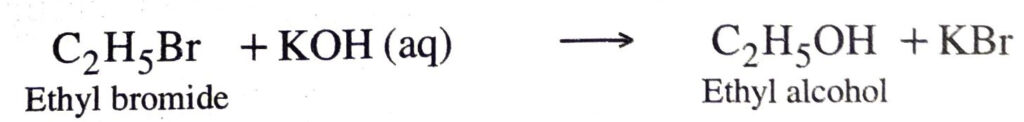
Q. 6. How ethanol and ethanoic acid can be differentiated on the basis of their physical and chemical properties ?
Ans. Physical Properties
| Ethanol | Ethanoic acid |
|
1. It has a typical alcoholic smell.
2. It has burning taste.
|
1. It has vinegar-like smell.
2. It has sour taste.
|
Chemical Properties
| Ethanol | Ethanoic acid |
|
1. It has no action on litmus paper.
2. It has no action with sodium bicarbonate.
|
1. It turns blue litmus solution red.
2. It decomposes sodium bicarbonate producing carbon dioxide.
|
Q. 7. Discuss the important characteristics of covalent compounds.
Ans. The important characteristics of covalent compounds are as given ahead :
1. Covalent compounds consist of molecules. Covalent compounds do not have any ions. Therefore, they consist of molecules. For example, H2, Cl2, H2O, NH3 etc.
2. Covalent compounds are liquids or gases in nature. Only a few covalent compounds are solids (e.g. sugar, glucose, iodine). These are mostly liquids (water, ethyl alcohol) or gases (oxygen, hydrogen, ammonia) at room temperature. Actually, the attractive forces in covalent molecules are weak and these molecules are not as close to one another as the ionic solids.
3. Covalent compounds have low melting and boiling points. As covalent molecules do not have ions, the attractive forces among them are weak. Therefore, the covalent molecules can be easily separated from each other. In other words, they have low melting and boiling points.
4. Covalent compounds do not conduct electricity. Covalent compounds normally do not conduct electricity. Some of them are poor conductors of electricity. The current is carried by the ions. As covalent compounds do not have ions, these are poor conductors of electricity.
5. Covalent compounds are insoluble in water. Covalent compounds generally do not dissolve in water. They are soluble in alcohol, ether, benzene etc. which are called organic solvents. However, some of them such as ammonia and ethyl alcohol are water soluble.
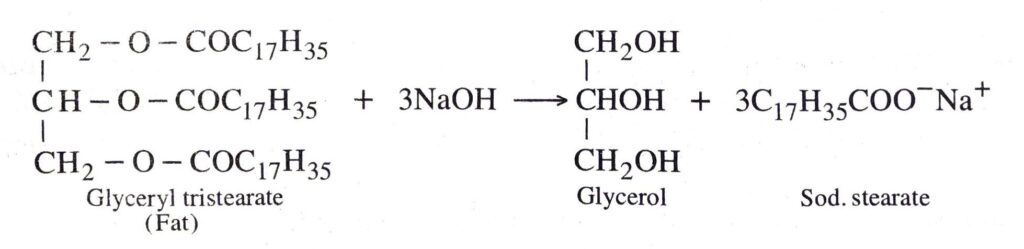
Q. 8. Describe a method for the preparation of soap.
Ans. Soaps are sodium or potassium salts of higher fatty acids. When the naturally occurring esters called fats or oils are heated with NaOH solution, they undergo hydrolysis to form sodium salt of higher fatty acid (called soap) and glycerol.
Preparation of soap. Heat fat or oil with sodium hydroxide solution. After a few minutes, and constant stirring, the oil and water layers get mixed.
Add 5-10 g of common salt to it, stir the mixture and allow it to cool. On cooling pale yellow a solid forms as a cake called soap.
The same principle is used for making soap in soap industry. Some other substances like perfumes, disinfectants and medicines are added to soap to give it desired characteristics.
Example :
Q. 9. Fill in the blanks :
1. The first member of alkanal series is ………….. .
2. Formation is an aqueous solution of ………….. .
3. Fermentation of sugar gives ………….. .
4. IUPAC name of (CH3)3 COH is ………….. .
5. The IUPAC name of acetone is ………….. .
6. The IUPAC name of formic acid is ………….. .
7. The organic acid obtained from vinegar is ………….. .
8. ………………. are used for making artificial perfumes.
9. Organic acids turn …………….. litmus solution ………….. .
10. The functional group present in ethanol is ………….. .
Ans. 1. Methanoic acid 2. Methanol 3. ethanol 4. 2-methyl propan-2-ol 5. Propanone 6. methanoic acid 7. Acetic acid 8. Esters 9. blue, red, 10. hydroxyl (-OH)
Q. 10. What are substitution and addition reactions ?
Ans. Substitution reactions. These are the reactions in which one or more H-atoms of a molecule are replaced by corresponding number of other atoms or groups.
Examples
Methane reacts with chlorine in the presence of sunlight to form chloromethane and hydrogen chloride
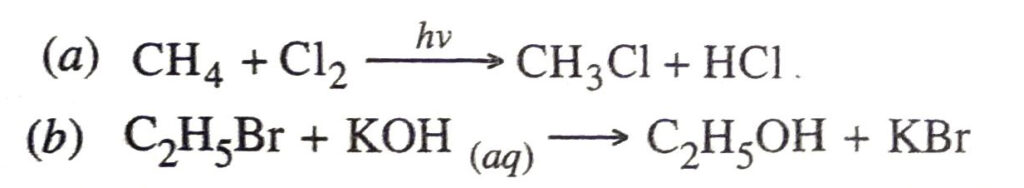
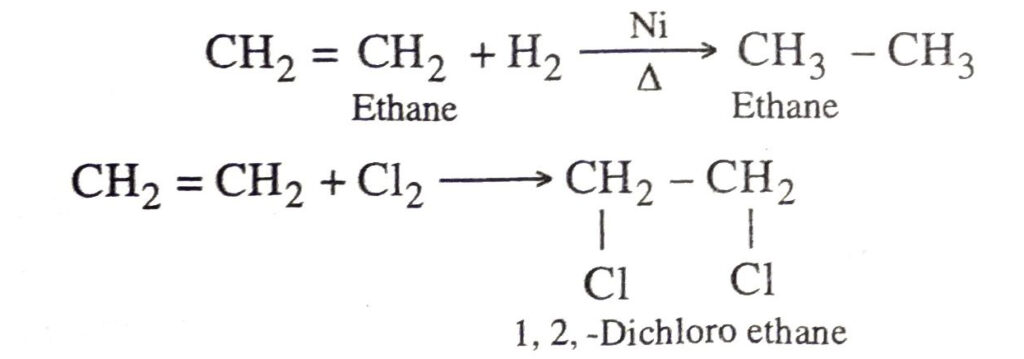
Addition reactions. These are the reactions in which an unsaturated hydrocarbon combines with another substance to give a single product.
Examples
Ethane reacts with hydrogen when heated in the presence of nickel catalyst to form ethane.
Q. 11. What are the two properties of carbon which lead to huge number of carbon compounds we see around us ?
Or
Explain versatile nature of carbon due to which it forms a large number of compounds.
Ans. The two properties of carbon which lead to a huge number of carbon compounds are :
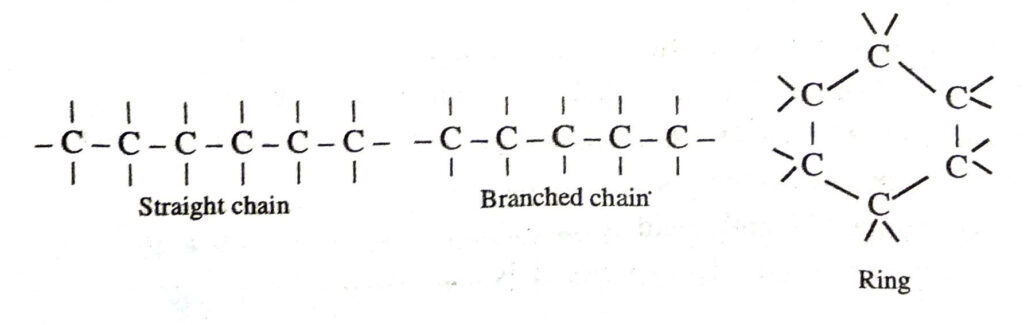
(a) Catenation. It is the property of carbon due to which a large number of carbon atom link among themselves to form long straight or branched or of different lengths. Also carbon atoms may be linked by single, double or triple bonds.
(b) Tetracovalency. Carbon shows tetracovalency. It requires 4 electrons to become stable. Therefore carbon can form four covalent bonds with carbon or some other atom. Such as hydrogen, oxygen, nitrogen, sulphur, halogen etc. This further increases the number of compounds of carbon.
Q. 12. Define Saponification. Draw a dot structure of carbon tetrachloride and write its formula.
Ans.
(i) Saponification. It is the process of alkaline hydrolysis of fat to give soap and glycerol.
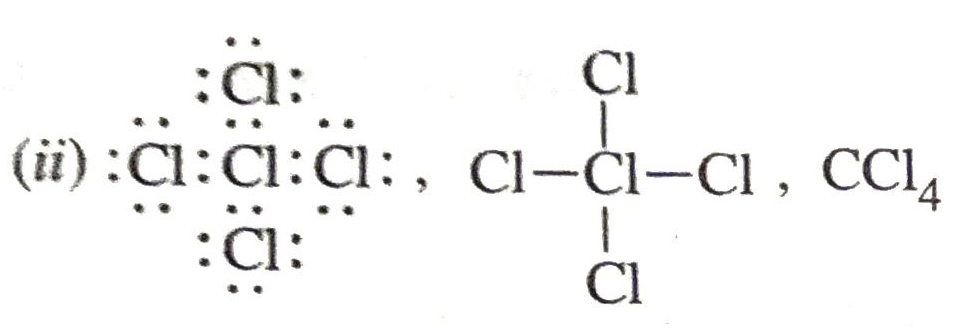
Q. 13. How would you bring about the following conversions ?
(a) Ethanol to ethane
(b) Propanol to propanoic acid. Ans.
Ans.
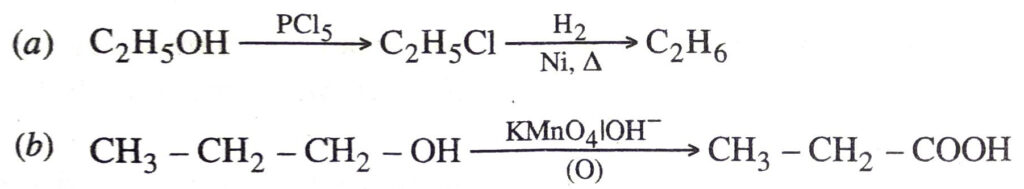
Q. 14. Give the properties of ethanol and ethanoic acid.
Or
How can ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties ?
Or
Give two chemical properties of ethanol.
Ans. Properties of ethanol
1. It is a colourless liquid.
2. It is soluble in water.
3. It produces hydrogen with sodium metal.
2C2H5OH + 2Na → 2C2H5O-Na+ + H2
4. It forms an ester with acetic acid.
Properties of ethanoic acid
1. It is a colourless liquid.
2. It has vinegar-like smell.
3. It is soluble in water.
4. It forms an ester with ethyl alcohol.
5. It produces hydrogen with sodium. 2CH3COOH + 2Na
2CH3COO + 2Na → 2CH3COONa + H2
6. It turns blue litmus solution red.
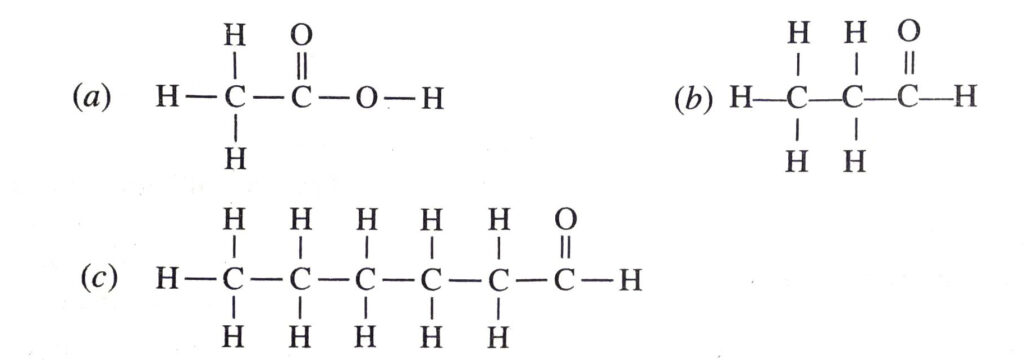
Q. 15. Write down the structures of the following compounds :
(a) Ethanoic acid
(b) Propanone
(c) Hexanol
Ans.
Q. 16. Discuss briefly the physical and chemical properties of ethanoic acid.
Or
How and under what condition does ethanoic acid react with ethanol and sodium carbonate ?
Ans. Physical properties of Ethanoic acid
1. It is a colourless liquid having a sour taste.
2. It has vinegar-like smell.
3. It boils at 391 K and freezes at 290 K.
4. It is miscible with water in all proportions.
Chemical properties of Ethanoic acid
1. Esterification. When an acid is heated with an alcohol in the presence of conc. H2SO4 an ester is produced. This is a slow reversion reaction and is called esterification.
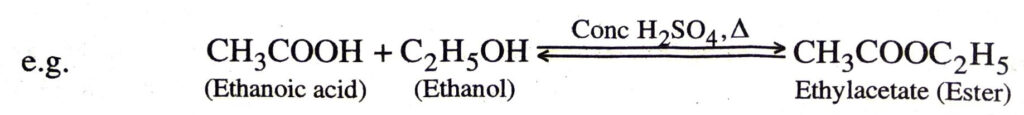
Esters are sweet-smelling substances.
2. Action with a base
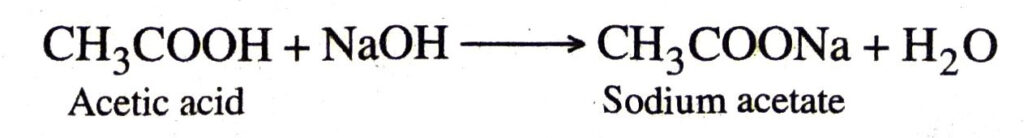
3. Action with carbonates and bicarbonates. Ethanoic acid decomposes carbonates and bicarbonates producing salt, carbon dioxide and water.
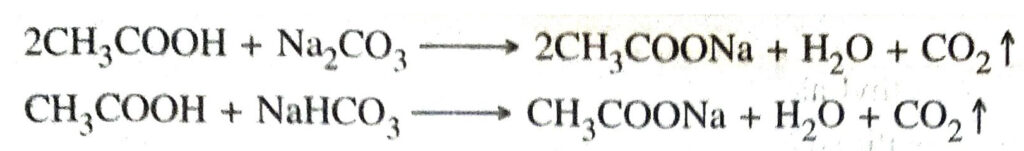
4. Action with Na Sodium
2CH3COOH + 2Na ↓ 2CH3COONa+ H2
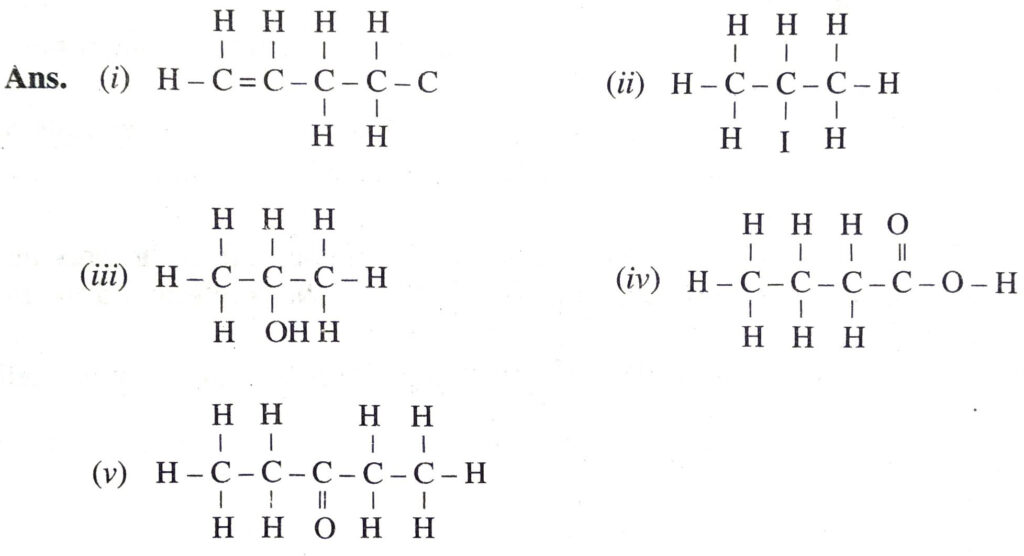
Q. 17. Draw the structures for the following compounds :
(i) 1-Butene
(ii) 2-Iodopropane
(iii) 2-Propanol
(iv) Butanoic acid
(v) 3-Pentanone.
Q. 18. Discuss briefly the physical and chemical properties of Ethanol.
Ans. Physical Properties :
(i) It is colourless liquid having typical alcoholic smell.
(ii) It has burning tast.
(iii) It is soluble in water.
Chemical Properties :
(i) It has no action on litmus paper.
(ii) It has no action with sodium bicarbonate.
(iii) On heating with sodium metal, it gives colourless and odourless hydrogen as :
(iv) On heating with acetic acid, it forms ester having fruity smell.
SHORT ANSWER TYPE QUESTIONS
Q. 1. Fill in the blanks :
(a) Two atoms of the same element combine to form a molecule. The bond between them is known as ……………… bond.
(b) In the formation of oxygen molecule, the oxygen atoms share …….. electrons each.
(c) The number of single covalent bond in the molecule of ammonia is ………………
Ans. (a) covalent (b) two (c) three.
Q. 2. What happens when ethanol is warmed with ethanoic acid in the presence of few drops of concentrated sulphuric acid ? Give equation of the reaction and the name of the product formed.
Ans. When ethanol reacts with ethanoic acid on warming with conc. H2SO4, sweet smelling ester is formed.
The name of the product is ethyl ethanoate.
Q. 3. Why do covalent molecules have definite shapes ?
Ans. A covalent bond is formed by the sharing of electrons between the atoms. The bonds are represented by dashes (-) and are directional in nature. The directional nature of the covalent bonds gives covalent molecules having two or more such bonds of a definite shape. This is also called definite geometry of the covalent molecule. Thus, the geometry of a covalent molecule may be defined as the arrangement of the different atoms with respect to each other.
Q. 4. Where do compounds of carbon find applications ?
Ans. The carbon compounds are being increasingly used as a source of energy, as medicines, colours, textiles, plastics, food preservatives etc.
Q. 5. Give chemical tests to :
(a) detect the presence of ethanol.
(b) show that methanal contains an aldehyde group.
Ans. (a) Warm the compound with ethanoic acid and a few drops of conc. H2SO4. A sweet smell (due to the formation of ester) indicates the presence of ethanol.
(b) Methanal gives silver mirror test with Tollen’s reagent. This shows that methanal contains aldehydic group.
Q. 6. (a) Define allotropy.
(b) Name three allotropic forms of carbon.
Ans. Allotropy. When an element exists in more than one form having different physical properties but same or slightly different chemical properties, the various forms are called allotropic forms or allotropes and this phenomenon is called allotropy.
The element like carbon, sulphur, phosphorus etc show allotropy.
The three allotropic forms of carbon are:
(i) Diamond (ii) Graphite (iii) Fullerenes.
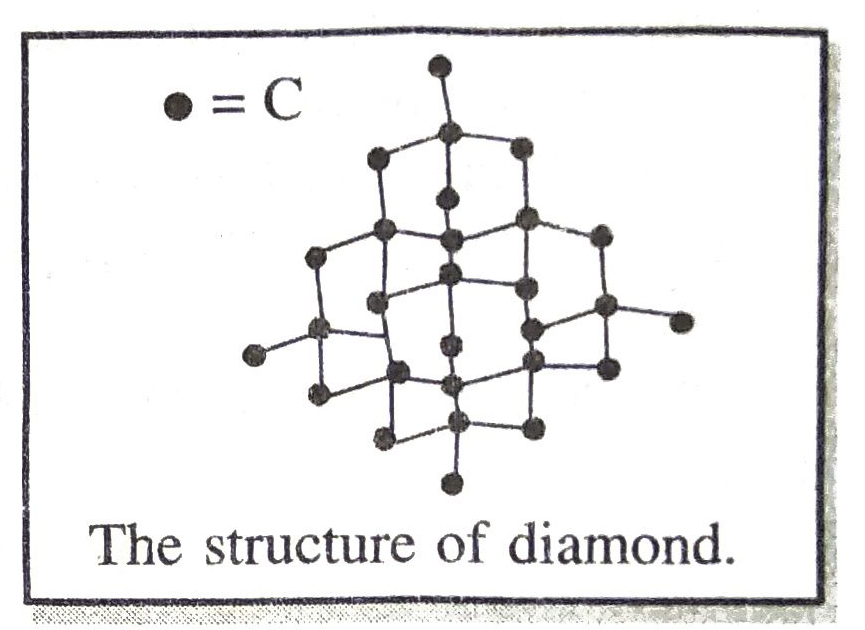
Q. 7. Give the structure of diamond and explain one property based upon structure.
Ans. The structure of diamond is as shown below :
In diamond, each carbon atom is bonded to four other carbon atoms by covalent bonds resulting in the formation of a three dimensional network structure.
Due to the presence of strong covalent bonds, diamond is hard and has high melting point.
Q. 8. Give the structure of graphite and explain its one property based upon structure.
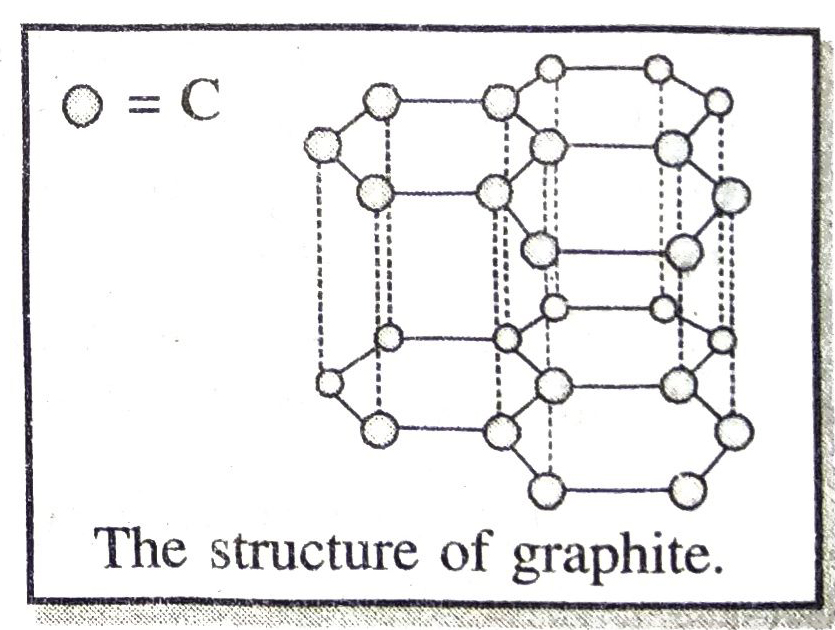
Ans. The structure of graphite is as shown below :
In graphite each C-atom is bonded to three other carbon atoms to four hexagonal rings which are held together by weak van der Waals forces of attraction. Therefore, graphite has a two dimensional sheet like structure.
Due to the presence of one free electron left with each C-atom, graphite is a good conductor of electricity.
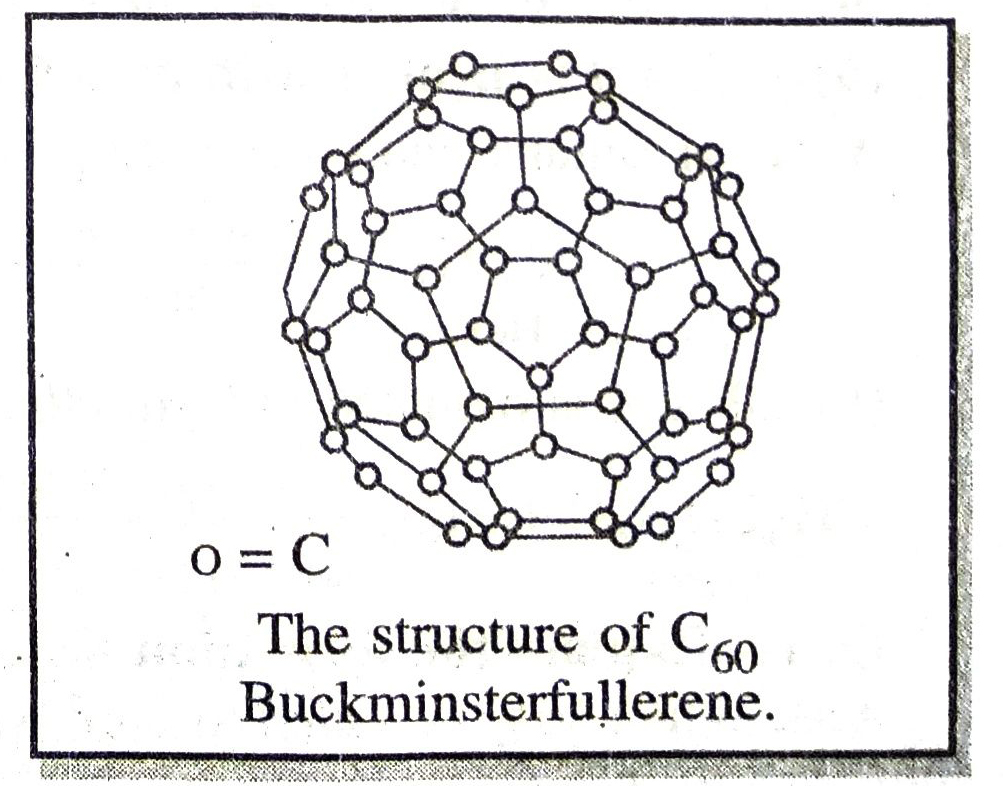
Q. 9. Give the structure of Fullerenes.
Ans. Fullerenes are also allotropes of Carbon, for example, the fullerene, C60 has carbon atoms arranged in the form of a football and it looked like the geodesic dome designed by the US architect Buckminster Fuller, the molecule was named fullerene.
Q. 10. What is a Detergent ? Name one detergent. Why have detergents replaced soap as a washing agent ?
Ans. Detergents. These are sodium alkyl benzene sulphonates or sodium alkyl sulphates. Example: Sodium lauryl sulphate,
Detergents have replaced soap as a washing agent because unlike soap they can be used for washing purposes in cold water, acidic medium, hard water and have better cleansing action as compared to soap.
Q. 11. Why is a mixture of water and alcohol used instead of water in radiators of vehicles in cold countries? Give two reasons.
Ans. It is used as antifreeze because it has freezing point much below 0°C. If ethanol is not added to water, it will freeze in radiators. Mixture of water and alcohol does not corrode the material of the radiator.
Q. 12. Write two tests to show that acetic acid is acidic in nature.
Ans. (a) Add blue litmus paper into solution of acetic acid, it will turn red showing acidic nature.
(b) Add sodium bicarbonate. If there is brisk effervescence due to CO2, it proves acidic nature.
Q. 13. Give any four uses of ethyl alcohol in daily life.
Ans. (a) It is used as an antiseptic to sterlize wounds and syringes in hospitals. (b) It is used as an antifreeze in car radiators. (c) It is used as fuel in spirit lamps and internal combustion engines. (d) It is used in making alcoholic drinks e.g. whisky, wine, beer, etc.
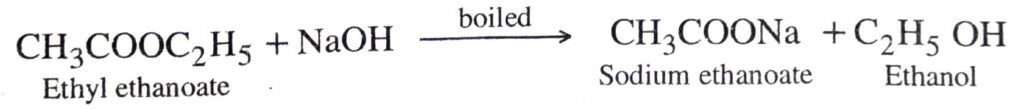
Q. 14. What is saponification? Give an example.
Ans. When an ester reacts with water in presence of a base, a salt of carboxylic acid and an alcohol are produced. Such a reaction is called saponification. e.g. when ethyl ethanoate is boiled with a solution of sodium hydroxide, sodium ethanoate and ethanol are produced.
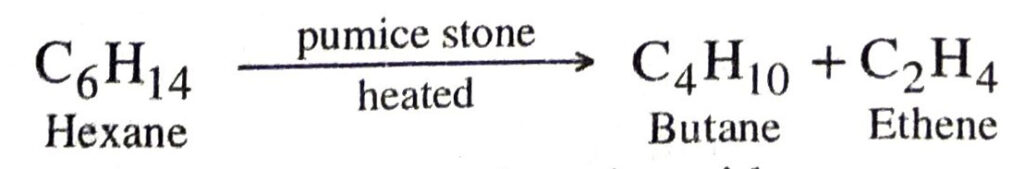
Q. 15. What happens when :
(a) Vapours of hexane are passed over heated pumice stone.
(b) Ethanol is heated with an acidic solution of potassium dichromate.
Ans. (a) Butane and ethene are produced.
(b) Ethanol is oxidised to form ethanoic acid.
Q. 16. Explain the formation of scum when hard water is added to soap.
Ans. When soap is added to hard water, calcium and magnesium salts present in it react with soap to form scum.
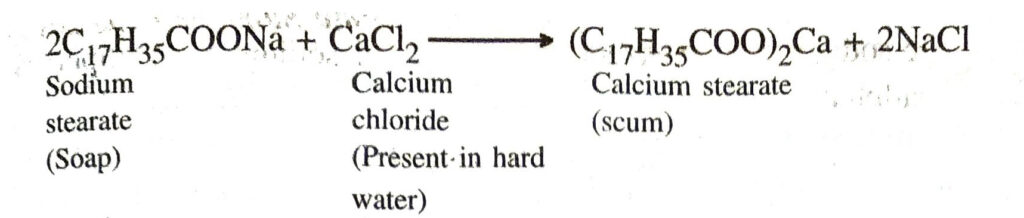
Q. 17. What are the advantages of synthetic detergents over soaps ?
Or
Explain why soaps are not effective cleansing agents in hard water.
Or
Why detergents are preferred over soap ?
Ans. Advantages of synthetic detergents over soaps :
(a) Synthetic detergents can be used even with hard water also whereas soap cannot be used in hard water because soaps don’t produce lather readily with soap.
(b) Synthetic detergents are more soluble and have better cleansing action than soaps.
(c) Synthetic detergents can even be used with acidic water whereas soaps cannot be used.
Q. 18. Why excessive use of synthetic detergents is discouraged ? Give reasons.
Ans. Disadvantages of synthetic detergents over soaps
(a) Synthetic detergents are non-biodegradable and, therefore, can cause water pollution in lakes and rivers. This affects the fish and other aquatic organisms (because phosphatic salts present in detergents can cause rapid growth of algae in water bodies which further leads to decrease in the dissolved oxygen) resulting in the death of certain aquatic organisms.
(b) Synthetic detergents are also known to affect the texture and colour of the fabrics.
Q. 19. What is the common name of ethanoic acid ? How ethanoic acid is different from vinegar? Give the use of vinegar in our daily life.
Ans. Ethanoic acid (CH3COOH) is commonly known as acetic acid. The dilute solution of acetic acid in water (6-8%) is known as vinegar. The vinegar is used for preserving food, sausage, pickles, etc.
Q. 20. Give two uses of ethanol.
Ans. (a) It is used as a solvent.
(b) It is used to prepare alcoholic drinks.
Q. 21. How vinegar is prepared from ethanol ? Give reaction to support your answer.
Ans. Vinegar is prepared by the oxidation of ethanol in air, in the presence of acetobacter enzyme.
Q. 22. What is meant by hydrolysis of an ester ? Explain.
Ans. On treating with an alkali solution (sodium hydroxide) the ester is converted back to the constituent alcohol and sodium salt of the acid.
This reaction is hydrolysis of an ester. It takes place in presence of alkali and is known as saponification.
Q. 23. Differentiate between soaps and synthetic detergents.
Ans.
| Soaps | Synthetic detergents |
|
1. Soaps are sodium or potassium salts of higher fatty acids e.g sodium stearate.
2. Soaps are prepared from vegetable oils, animal and fats.
3. Soaps have relatively weak cleansing action.
4. Soaps form curdy white precipitate with calcium and magnesium salts present in hard water and hence, are not used in hard water.
5. Soaps cannot be used in acidic medium as they are decomposed into carboxylic acids in acidic medium.
6. Soaps do not cause water pollution.
|
1. Synthetic detergents are sodium alkyl sulphates or sodium alkyl benzene sulphonates e.g. sodium n-dodecylsulphate.
2. Synthetic detergents are prepared from the hydrocarbons obtained from petroleum.
3. They have strong cleansing action.
4. Calcium and magnesium salts of detergents are soluble in water and, therefore, no curdy white precipitates are obtained in hard water and hence synthetic detergents can be used even in hard water.
5. They can be used in acidic medium as they are the salts of strong acids and are not decomposed in acidic medium.
6. Synthetic detergents cause water pollution.
|
Q. 24. Why are detergents preferred over soap ?
Ans. 1. Detergents have more cleansing power.
2. These can be used in hard water.
3. These can be used even in cold water.
4. They can be used in acidic medium.
Q. 25. Give the structural isomers of pentane.
Ans. These are :
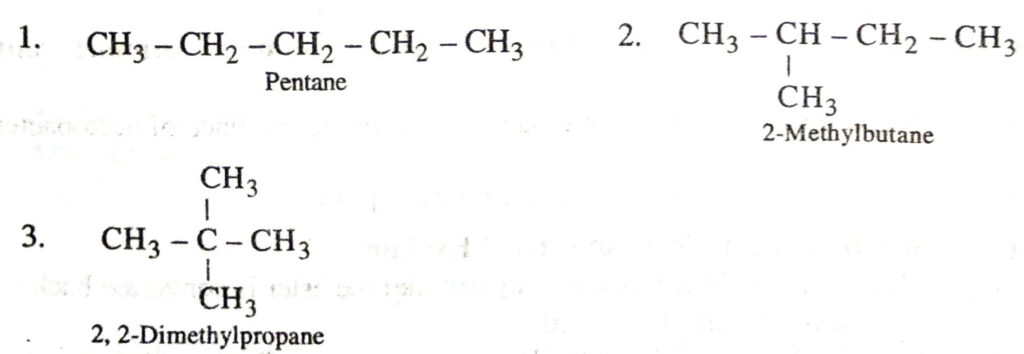
Q. 26. Where do compounds of carbon find applications ?
Ans. Carbon compounds are used in large number of industries such as those of food, fruits, paints, varnishes, sugar, paper, plastic etc.
Q. 27. Explain the versatile value of carbon.
Ans. Carbon has atomic number 6.
It has 4 electrons in its valence shell (2, 4).
Carbon can form a large number of compounds due to :
(a) Its tetracovalency
(b) Catenation.
Carbon forms both saturated and unsaturated compounds. It can form open chain as well as ring compounds.
1. All the organic compounds contain carbon. Carbon and its compounds are major sources of fuel.
2. The compounds of carbon are used in daily life, laboratories and industries.
Q. 28. Define homologous series. Give its characteristics.
Or
What is Homologous series? Explain with an example. State characteristics of a homologous series.
Ans. A series of compounds having similar structural formulae, same functional group and hence similar chemical properties is called a homologous series.
For example, homologous series of aldehydes (or alkanals) can be represented as :
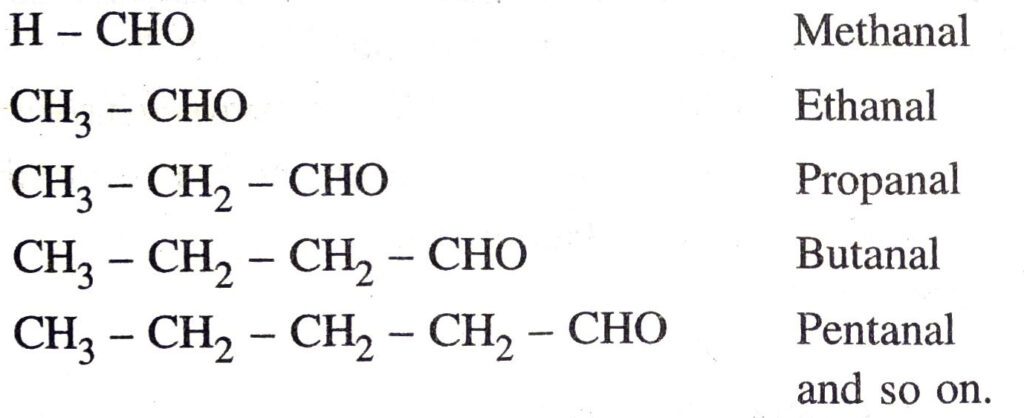
Characteristics
1. Any two adjacent members of a homologous series differ by CH₂ unit in their molecular formula.
2. Each homologous series has a general formula.
3. The members of a homologous series have the same functional group.
4. The members of a homologous series have similar chemical properties.
Q. 29. Which of the following will give addition reactions and why?
C4H10, C2H6, C2H4, CH4, C3H8, C3H4.
Ans. C2H4 (C ≡ CH) and C3H4 (CH3—C ≡ CH) will give addition reactions because they contain multiple bonds.
Q. 30. Explain the following terms:
(i) Esterification (ii) Decarboxylation.
Ans. (i) Esterification. The reaction of carboxylic acid with an alcohol in the presence of concentrated sulphuric acid to form esters is called esterifications.
(ii) Decarboxylation. The reaction in which carbon dioxide is removed from a of carboxylic acid is known as decarboxylation. molecule
Q. 31. What is meant by denatured alcohol ? What is the need to denature alcohol?
Ans. Denatured alcohol is 95% ethanol. To avoid misuse of ethanol for drinking purposes, it is made unfit by mixing it with some poisonous substances such as methanol, pyridine, copper sulphate etc. This process is called denaturing of alcohol and the alcohol thus formed is called denatured alcohol.
Q. 32. Compare the following properties of ethanol and ethanoic acid.
1. Litmus test
2. Sodium metal reaction
3. Sodium bicarbonate test.
Ans.
| Test | Ethanol | Ethanoic acid |
|
1. Litmus test
2. Sodium metal test
3. Sodium bicarbonate test
|
No effect
H₂ gas is evolved
No reaction
|
Turns blue litmus red
H₂ gas is evolved
Brisk effervescence due to the evolution of CO₂.
|
Q. 33. Explain why are soaps not effective cleansing agents in hard water?
Ans. Hard water contains Ca2+ and Mg2+ ions which react with soap molecules forming insoluble precipitates called scum. Due to this reason, soap is not able to form lather and cannot do the cleansing action. In this way, soap gets wasted when used with hard water.
Q. 34. Give important uses of ethanoic acid.
Ans. Uses of ethanoic acid
1. It is used in the manufacture of dyes, perfumes and rayon.
2. It is used in the manufacture of plastics, rubber and silk industries.
3. It is used as a solvent.
4. It is used as a vinegar in cooking, as food dressing and for preparing pickles.
5. It is used for the manufacture of chemicals like acetone, acetic anhydride, etc.
VERY SHORT ANSWER TYPE QUESTIONS
Q. 1. Name two covalent compounds which are water soluble.
Ans. Ammonia (NH3), Glucose (C6H12O6).
Q. 2. Give the name and the formula of a compound having one double bond and four single bonds.
Ans. The compound is ethylene, C2H4 and its structural formula is
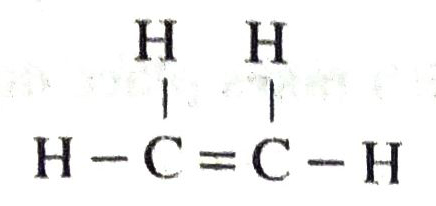
Q. 3. What is the necessary condition for the formation of a covalent compound between two atoms of an element to form a molecule ?
Ans. The covalent bond is generally formed between the atoms of non-metals and both the combining atoms are short of electrons than the stable nearest noble gas configurations in their outermost orbits.
Q. 4. Write the two possible compounds of molecular formula C3H6O.
Ans. CH3CH2CHO : Propanal
CH3COCH3 : Propanone.
Q. 5. What is rectified spirit ?
Ans. Ethanol containing five per cent methanol is known as rectified spirit.
Q. 6. Write the chemical equation for combustion of ethanol.
Ans. CH3CH2OH + 3O2 → 2CO2 + 3H2O
Q. 7. Complete the reaction :
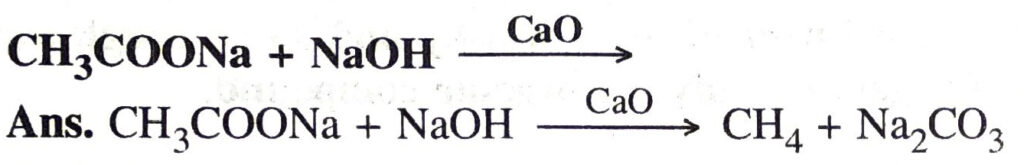
Q. 8. The general formula of carboxylic acid is C1 H2n +1—COOH. Name the carboxylic acid having n = 1 and give its molecular formula.
Ans. Acetic acid CH3COOH.
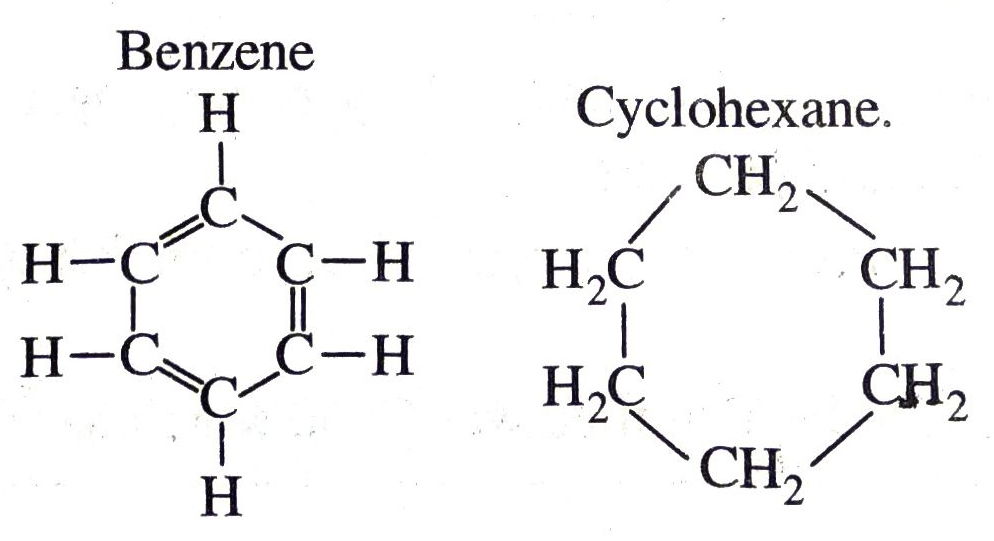
Q. 9. Draw the structure of benzene and cyclohexane.
Ans.
Q. 10. An organic compound ‘A’ has the molecular formula CH2O2. It turns blue litmus red and gives brisk effervescence with NaHCO3. Identify ‘A’ and give chemical reaction.
Ans. A is methanoic acid, HCOOH. It turns blue litmus red.
HCOOH + NaHCO3 → HCOONa + CO2 +H2O
Q. 11. An organic compound X of molecular formula C2H6O, on oxidation with potassium dichromate and concentrated sulphuric acid, produces acetic acid (CH3COOH). What is the compound X. Write the equation for the reaction.
Ans. X is ethyl alcohol (ethanol) i.e. C2H5OH
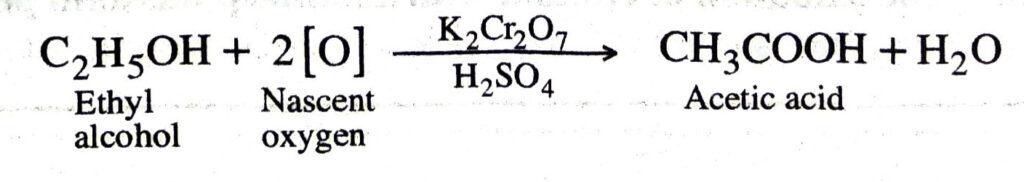
Q. 12. Write the molecular formula of the fourth and the fifth members of the homologous series of carbon compounds represented by the general formula CnH2n + 1 — OH.
Ans. (a) Fourth member: C4H9OH
(b) Fifth member: C5H11OH
Q. 13. Write the chemical equation for the reaction which takes place during burning of ethanol in air.
Ans. CH3CH2OH + 3O2 → 2CO2 + CH2O
Q. 14. Write the molecular formula of the acid from which the ester (whose formula is given below) has been derived :
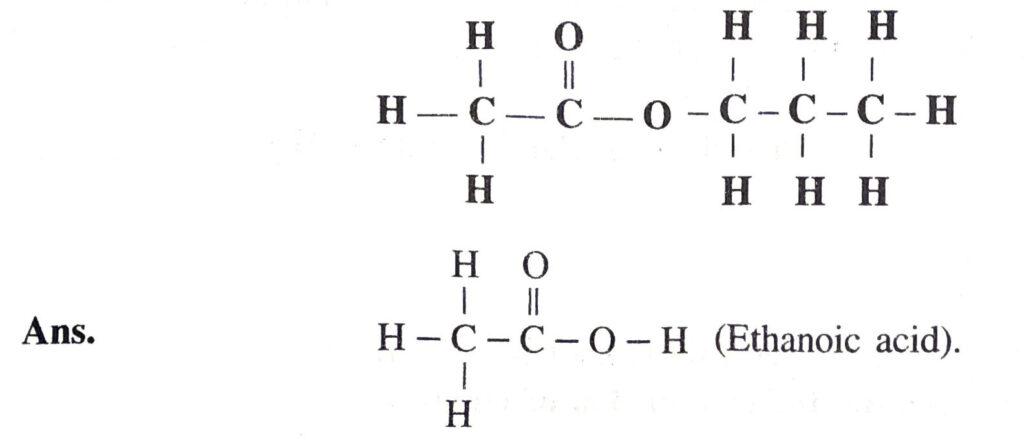
Q. 15. Why do some people add common salt during the preparation of soap ?
Ans. Common salt is added to precipitate out soap completely from oil or fat.
Q. 16. An organic compound is a constituent of beer, whisky and some cough syrup. It is produced by the fermentation of sugar. Identify the organic compound.
Ans. Ethanol.
Q. 17. Why graphite is a good conductor of electricity?
Ans. This is due to the presence of free or mobile electrons.
MULTIPLE CHOICE QUESTIONS
Select the correct answer out of the four alternatives :
1. Butanone is a four carbon compound with the functional group :
(A) carboxylic acid
(B) aldehyde
(C) ketone
(D) alcohol.
Ans. (C) ketone
2. Ethane (C2H6) has :
(A) 6 covalent bonds
(B) 7 covalent bonds
(C) 8 covalent bonds
(D) 9 covalent bonds.
Ans. (B) 7 covalent bonds
3. Propanone has :
(A) 7 covalent bonds
(B) 8 covalent bonds
(C) 9 covalent bonds
(D) 10 covalent bonds.
Ans. (D) 10 covalent bonds.
4. Soap molecule forms:
(A) Oil droplets
(B) Dirt molecules
(C) Miscelles
(D) All.
Ans. (C) Miscelles
5. n-Pentane and isopentane represent :
(A) Structural isomers
(B) Homologues
(C) Same compound
(D) None of these.
Ans. (A) Structural isomers
6. Any two adjacent members of a homologous series differ by :
(A) CH3 unit
(B) CH2 unit
(C) CH unit
(D) C2H4 unit.
Ans. (A) CH3 unit
7. The preparation of Vanaspati Ghee is an example of :
(A) Combustion
(B) Substitution reaction
(C) Addition reaction
(D) Oxidation.
Ans. (C) Addition reaction
8. Which of the following types of medicines are used for treating in digestions?
(A) Antibiotic
(B) Analgesic
(C) Antacid
(D) Antiseptic.
Ans. (C) Antacid
9. Carboxylic acid group is present in one of the following:
(A) Propanone
(B) Acetaldehyde
(C) Ethanoic acid
(D) Hydrochloric acid.
Ans. (C) Ethanoic acid
10. Organic acids have the functional group :
(A) – OH
(B) – COOH
(C) – CHO
(D) >C=O
Ans. (B) – COOH
11. Aldehyde group is present in one of the following:
(A) Propanone
(B) Acetaldehyde
(C) Ethanoic acid
(D) Hydrochloric acid.
Ans. (B) Acetaldehyde
12. The hydrocarbon part of soap is :
(A) Insoluble in water
(B) Insoluble in oil
(C) Insoluble in greese
(D) None of these.
Ans. (A) Insoluble in water
Follow on Facebook page – Click Here
Google News join in – Click Here
Read More Asia News – Click Here
Read More Sports News – Click Here
Read More Crypto News – Click Here