WBBSE 10th Class Science Solutions Physical Science & Environment Chapter 1 Environmental Concern
West Bengal Board 10th Class Science Solutions Physical Science & Environment Chapter 1 Environmental Concern
WBBSE 10th Class Physical Science & Environment Solutions
Synopsis
Atmosphere
- Atmosphere: The gaseous layer surrounding the surface of earth that extends up to about 1600 km, is known as the atmosphere.
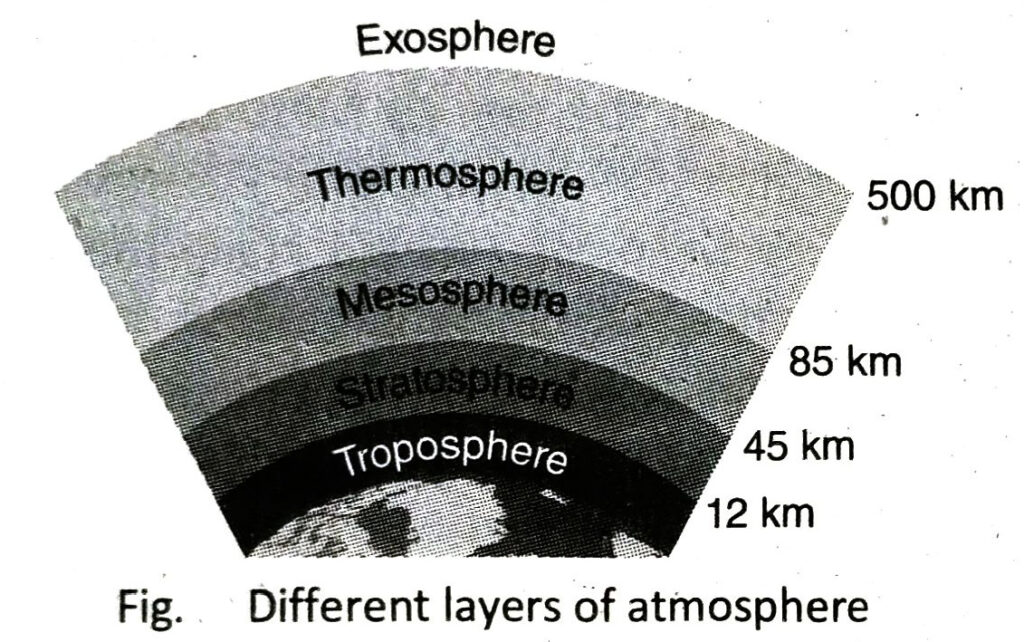
- Different layers of atmosphere: On the basis of temperature and pressure, atmosphere is divided into five different layers namely troposphere, stratosphere, mesosphere, thermosphere and exosphere.
- Convection current: On heating, fluids (liquids and gases) undergo expansion and hence their density decreases. So, the heated liquid or gas becomes lighter and moves upward. On the other hand, the cool heavy part of the upper region comes down. This results in the formation of circular current which is known as convection current.
- Wind and storm: Wind blows from a region of comparatively higher pressure to á region of comparatively lower pressure. Earth surface, under certain circumstances gets heated up. Hence the air adjacent to this earth surface also gets heated up and moves upward. This creates a low pressure zone in that region. Cooler air from surroundings rushes towards that region. Greater the difference in air pressure, faster the movement of air from the higher to lower pressure region. Thus wind is created. When wind blows above a specified speed, it is termed as storm.
Ozone Layer, Greenhouse Effect and Global Warming
- Ozonosphere: The harmful UV rays of the Sun are absorbed by the ozone layer or ozonosphere present in the stratosphere. In this way, the ozone layer protects living organisms from their harmful effects.
- Ozone hole: Increased use of chemicals and technology for the development of civilization has adverse effects on the ozone layer. Due to the constant decomposition of ozone molecules, ozone holes are found to be formed at different parts of the ozone layer.
- Compounds responsible for ozone layer depletion: Chlorofluorocarbons (CFCs) and oxides of nitrogen (mainly NO) are mainly responsible for the depletion of ozone layer.
- Greenhouse effect: Gases like CO2, CH4, N2O, CFC, water vapour traps the retransmitted infrared radiations of higher wavelengths. This helps to keep the earth surface warm and thus a favorable environment is created for the survival of living beings. This is called the greenhouse effect.
TOPIC – A
Atmosphere
SHORT AND LONG ANSWER TYPE QUESTIONS
Q.1 What is atmosphere? What are the different regions of the atmosphere?
Ans. The gaseous layer surrounding the surface of earth that extends up to about 1600 km, is known as the atmosphere. Under the influence of gravity, this surrounding remains attached to the surface of the earth.
On the basis of height and temperature, the atmosphere is divided into five different regions. There are as follows-
| Layers |
Height from the earth surface |
Temperature Range (°C) |
| Troposphere |
0 – 12 km |
+15 to -60 (temperature increases with the increase of height) |
| Stratosphere |
12 – 45 km |
-60 to 0 (temperature increases with the increase of height) |
| Mesosphere |
45-85 km |
0 to 100 (temperaincreases with the increase of height) |
| Thermosphere |
85-500 km |
-100 to +1200 (temperature increases with the increase of height) |
| Exosphere |
500-1000 km |
> 1200 |
Q.2 What are ‘homosphere’ and ‘heterosphere’ of the atmosphere?
Ans. The lower part of the atmosphere (extends up to an approximate height of 85 km above the earth surface) is homogeneous in nature. The gaseous components (N2, O2, Ar, CO2, water vapour etc.) are evenly distributed in this region. This part of the atmosphere is called homosphere.
The remaining part of the atmosphere above the homosphere where the components are unevenly distributed, is called heterosphere. This part starts just above the homosphere region and extends between 85km above the earth’s surface up to a distance of 1000 km.
Q.3 Define troposphere. Why is it called re. Why is it ca ‘turbulent sphere’?
Ans. The lowest layer of the atmosphere is called the troposphere. It extends from the sea level up to a height of about 12 km.
This layer contains dust particles, water vapour, clouds etc. Different natural phenomena like storms, rains, lightning, thunderstorms etc., occur in this region. So, it is also known as the turbulent sphere.
Q.4 What is stratosphere? Why is it dynamically stable?
Ans. The atmospheric layer that extends up to a height of 45 km above the troposphere is known as stratosphere.
There is very little air in this region and dust particles, water vapour etc. are absent in this layer. Hence, this layer is free of several associated turbulence like clouds, rains, lightnings, thunderstorms etc. Thus, it is dynamically stable.
Q.5 What is mesosphere? Name the top here? Name the fo layer of mesosphere.
Ans. The atmospheric layer present just above stratosphere and extends up to a height of 85 km below thermosphere is known as mesosphere.
The top layer of the mesosphere is known as mesopause. Temperature remains fixed (-92°C) in this region.
Q. 6 What is thermosphere? Why is it named ere? Why is it SO?
Ans. The atmospheric layer present just above mesosphere and extends up to a height of 500 km is known as thermosphere.
The temperature of this layer tends to increase abruptly with increasing altitude. At a height of about 120 km the temperature is about 500°C, at a height of 200 km the temperature becomes almost 700°C and at 480 km the temperature is about 1232°C. Therefore, this layer is called thermosphere.
Q.7 What is ionosphere? Why is it named What is lono Why is so?
Ans. The particular portion of thermosphere which contains several gaseous ions is known as ionosphere.
Cosmic radiation, X-rays, gamma rays coming from the Sun ionizes the gases (mainly nitrogen and oxygen) of this region. As a result, this particular region contains large number of ions and free electrons. Due to high concentration of ions in this region, this is called ionosphere.
Q.8 What is meant by ‘aurora’? What are aurora borealls’ and ‘aurora australis’?
Ans. Cosmic radiations, X-rays, gamma rays coming from the sun ionizes the gases present in ionosphere (specific portion of thermosphere) and produces large number of ions as well as free electrons. These charged particles undergo interactions with the magnetic field of the earth. As a result of this interactions bright lights appear in the sky of polar regions. These polar lights or natural light displays in the earth’s sky is commonly known as aurora.
The aurora formed in the sky of north pole is known as aurora borealis and the aurora formed in the sky of south pole is called aurora australis.
Q.9 Give brief description of the exosphere layer of atmosphere.
Ans. The atmospheric layer situated at a height of more than 500 km with respect to the surface of earth is called exosphere. It extends up to an altitude of about 1000 km. The temperature in this region is more than 1200°C. This layer contains hydrogen and helium gases. Artificial satellites and space stations are located at this layer.
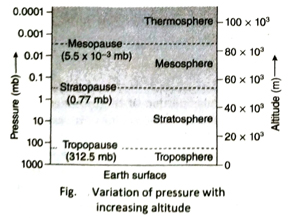
Q.10 How does atmospheric pressure change with the increase in altitude?
Ans. As we move up from the sea level, density of air decreases and hence, atmospheric pressure also decreases. Generally, with every 110 m rise in height, air pressure decreases by 1cm. However, the rate of change of pressure is not uniform at the upper layer of the atmosphere.
Q.11 Discuss how does temperature change in different layers of atmosphere as distance from the earth surface increases.
Ans. (1) The troposphere gets colder with the increase in altitude. With each km rise in altitude the temperature falls by 6.5°C. The top layer of troposphere has a temperature of about -56°C. (2) With increasing altitude, the temperature of stratosphere gradually increases and temperature becomes 0°C at the top. (3) Mesosphere is the coldest region of atmosphere. Temperature decreases as we move upward along this layer. (4) In thermosphere, temperature gradually increases with increasing height. This layer absorbs cosmic radiation coming from the Sun and is heated up. Consequently, its temperature reaches up to 1200°C. (5) The temperature of exosphere is higher than 1200°C.
Q.12 Why does temperature increase with increasing height at the stratosphere?
Ans. The oxygen molecules present in stratosphere is dissociated into atomic oxygen by absorbing the UV-rays of the sun. These oxygen atoms then combine with molecular oxygen to form ozone (O3) molecules. The last step is highly exothermic in nature and produces large amount of heat. As a result, the temperature of this layer increases gradually with increase in height and reaches at a temperature around 0°C at the top of the layer.
Q.13 What is convection? What is convection current?
Ans. Convection: The process by which the heated molecules of a liquid or a gas move from a region of higher temperature to a region of lower temperature thus carrying heat energy, is called convection.
Convection current: When a liquid or gas is heated, its density decreases due to expansion. The heated liquid or gas thus becomes lighter and moves upward. Comparatively colder and heavier liquid or gas from the surroundings then rushes towards that region to fill up the vacuum. Such circulation of gaseous or liquid layer results in the formation of convection current.
Q.14 How is convection current formed in air?
Ans. If the earth surface becomes hot the air adjacent to the earth’s surface also gets heated up. Hence it becomes lighter and rises up. This creates a low pressure zone in that region. Cold air from surroundings then rushes towards that region to fill up the vacuum. This creates convection current.
Q.15 Give two natural phenomena where 29 convection current is observed. When do they have maximum intensity?
Ans. Convection current is observed in land breeze and sea breeze.
Land breeze blows during the night and intensifies around the dawn. On the other hand, sea breeze blows at morning and intensifies around the dusk.
Q.16 Describe the formation of land breeze.
Ans. At night, the landmass in the coastal area loses heat at a faster rate than the sea water. Hence, the water remains comparatively warmer at night. Consequently, warm air above the sea becomes lighter and rises up while cold and dense air from the landmass starts moving towards the sea. This is known as land breeze.
Q.17 Describe the formation of sea breeze.
Ans. The specific heat of water is high, due to this the land in the coastal areas get heated up in a quicker manner compared to the sea water. As a result, the air above the landmass becomes lighter and rises up, thereby creating a low pressure zone. The cold, denser air from the sea then flows into this region resulting in sea breeze.
Q.18 How is storm formed?
Ans. Wind blows from a higher pressure region to a lower pressure region. The earth surface under certain circumstances get heated up so quickly that the air adjacent to the earth surface becomes warmer and lighter. Hence it goes upward creating a vacuum. As a result a low pressure zone is created in that region. The air from surroundings then rushes towards that region. Greater the difference in air pressure, faster the air blows from higher to lower pressure region. When wind blows above a specified speed, it is termed as storm.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. The layer of atmosphere having the highest density is
A. troposphere
B. stratosphere
C. mesosphere
D. thermosphere
Ans. A
2. The layer of atmosphere in which natural phenomena such as storms, rains, lightning occur is
A. thermosphere
B. troposphere
C. stratosphere
D. mesosphere
Ans. B
3. In atmosphere, the percentage (by mass) occupied by the troposphere is
A. 25
B. 50
C. 75
D. 60
Ans. C
4. The layer which is characterized by its calmness is
A. troposphere
B. stratosphere
C. mesosphere
D. thermosphere
Ans. B
5. The temperature at the topmost level of the stratosphere is
A. 50°C
B. -50°C
C. 0°C
D. -30°C
Ans. C
6. The atmospheric layer that absorbs ultraviolet rays is
A. troposphere
B. ozonosphere
C. thermosphere
D. mesosphere
Ans. B
7. The hottest layer of the atmosphere is
A. exosphere
B. ozonosphere
C. ionosphere
D. thermosphere
Ans. A
8. In which atmospheric region does aurora form?
A. Ozonosphere
B. lonosphere
C. Tropopause
D. Mesosphere
Ans. B
9. The atmospheric layers where temperature increases with increasing altitude are
A. troposphere and thermosphere
B. mesosphere and stratosphere
C. thermosphere and mesosphere
D. stratosphere and thermosphere
Ans. D
10. The atmospheric layer in which the radio waves get reflected is
A. stratopause
B. ozonosphere
C. ionosphere
D. mesosphere
Ans. C
11. For each kilometre rise in altitude from the earth’s surface, troposphere gets colder by
A. 5.6°C
B. 6.5°C
C. 3.5°C
D. 4.6°C
Ans. B
12. Artificial satellites and space stations are located at the
A. exosphere
B. thermosphere
C. mesosphere
D. stratosphere
Ans. A
13. Which layer of the atmosphere controls temperature and water cycle?
A. stratosphere
B. thermosphere
C. troposphere
D. mesosphere
Ans. C
14. Intensity of land breeze increases
A. around dawn
B. around noon
C. around dusk
D. at night
Ans. A
15. The layer of the atmosphere that can be called as ‘Natural Solar Screen’ is
A. troposphere
B. ozonosphere
C. thermosphere
D. exosphere
Ans. B
16. Several ions are present in
A. troposphere, stratosphere
B. troposphere, mesosphere
C. mesosphere, thermosphere
D. stratosphere, exosphere
Ans. C
17. The average height of troposphere from earth crust
A. 18 km
B. 15 km
C. 12 km
D. 20 km
Ans. C
18. In which layer of the atmosphere the amount of water vapour is the highest
A. troposphere
B. stratosphere
C. thermosphere
D. mesosphere
Ans. A
19. X-ray is absorbed in the layer called
A. troposphere
B. mesosphere
C. ionosphere
D. exosphere
Ans. C
20. Gases that are present in exosphere are
A. He, H2
B. N2 , O2
C. N2 , H2
D. H2 , O2
Ans. A
21. The coldest region of atmosphere is
A. stratosphere
B. exosphere
C. mesosphere
D. thermosphere
Ans. C
Answer in brief
1. State the range of troposphere in the polar region and equatorial region.
Ans. In the polar region, troposphere extends from sea level up to a height of 8-9 km and in the equatorial region it extends up to 16-18 km.
2. What is tropopause?
Ans. The narrow region, at a height of 12km from the earth’s surface, where troposphere meets stratosphere is known as the tropopause. In this region the temperature remains unaltered with the increase in height.
3. What is the air pressure at the lowest part of the troposphere?
Ans. The air pressure at the lowest part of the troposphere is equal to the pressure of 76 cm Hg column.
4. What is stratopause?
Ans. The thin layer between the stratosphere and mesosphere where the change in temperature becomes almost negligible is known as the stratopause.
5. Which part of the atmosphere is the coldest?
Ans. Mesosphere.
6. Which gases are predominantly found in thermosphere?
Ans. Nitrogen and oxygen.
7. The temperature gradually decreases at a definite rate as we move upward in the atmosphere from the earth’s surface. What is this phenomenon called?
Ans. Normal lapse rate.
8. What is the temperature at the upper end of the troposphere?
Ans. At the upper end of the troposphere, the temperature ranges between -56°C to -60°C.
9. Give examples of local winds
Ans. Land breeze and sea breeze.
10. Name the lowest layer of atmosphere.
Ans. Troposphere.
11. In which layer of the atmosphere do storm and rain occur?
Ans. Troposphere.
12. The living world of the earth crust remain in direct contact with which layer of the atmosphere?
Ans. Troposphere.
13. In which layer of the atmosphere does the strongly moving ‘Jet stream’ flow?
Ans. The Jet Stream’ flows in the tropopause region.
14. Through which layer of the atmosphere can a jet plane fly?
Ans. Jet planes can fly through stratosphere.
15. How does the temperature change with increase in height of the stratosphere?
Ans. The temperature increases with increase in height in the stratosphere.
16. Name some gaseous components present in stratosphere?
Ans. Nitrogen, oxygen and ozone.
17. What is the temperature of the mesopause part of mesosphere?
Ans. -92°C.
18. Aurora Borealis is found in which region of the atmosphere?
Ans. lonosphere region of thermosphere.
19. Which layer is used for communication with the help of radio waves?
Ans. lonosphere of thermosphere.
20. Mention the maximum temperature of thermosphere.
Ans. Almost 1200°C.
21. Which layer of the atmosphere is suitable for the installation of artificial satellites?
Ans. Exosphere.
Fill in the blanks
1. …….. is the most dense layer of the atmosphere.
Ans. Troposphere
2. The layer separating troposphere and stratosphere is called ……….
Ans. tropopause
3. When a meteorite enters the earth’s atmosphere, it burns due to it’s collision with the air of ………. layer.
Ans. mesosphere
4. The lower part of ………… is known as ionosphere.
Ans. thermosphere
5. Due to the presence of excess amount of ……….. gas in the stratosphere, the temperature of this region rises with altitude.
Ans. ozone
6. As the distance from the earth surface increases, the ……….. of air decreases.
Ans. density
7. ……….. breeze helps in sailing ships offshore.
Ans. Land
8. The term troposphere was first used by the scientist ……….
Ans. Teisserenc de Bort
9. The word troposphere means …………. region.
Ans. turbulent
10. The temperature of the troposphere ………….. with increases in height.
Ans. decreases
11. The air layer above the troposphere is called …………
Ans. stratosphere
12. The temperature of stratosphere ………….. with increase in height.
Ans. increases
13. The layer of the atmosphere in which Aurora can be seen is …………
Ans. thermosphere
14. The effect of charged molecules in the ionosphere of atmosphere results in the formation of ………..
Ans. Aurora
15. Water cycle of the earth is controlled by ………….
Ans. troposphere
State whether true or false
1. In troposphere, with increase in height, the pressure decreases.
Ans. True
2. Stratosphere is the most dense layer of the atmosphere.
Ans. False
3. Convection currents are formed in solid, liquid and gaseous states of matter.
Ans. False
4. Heterosphere starts from a height of 85 km. relative to the surface.
Ans. True
5. Air pressure decreases with increase in height in troposphere.
Ans. True
6. Water vapour, clouds etc. do not exist in troposphere.
Ans. False
7. Due to the presence of dust particles in troposphere, the sky looks blue.
Ans. True
8. Aurora can be seen in ozonosphere.
Ans. False
9. The maximum temperature of thermosphere is around 1200°C.
Ans. True
10. Atmospheric pressure in Darjeeling is greater than that in kolkata.
Ans. False
TOPIC – B
Ozone Layer, Greenhouse Effect and Global Warming
SHORT AND LONG ANSWER TYPE QUESTIONS
Q.1 What is ozonosphere? Why is this layer so essential for us?
Ans. Stratosphere contains an ozone gas layer which is extended from 16 km to 30 km with respect to the earth’s surface and the concentration of ozone gas is maximum in this layer. This particular layer is called ozonosphere.
The harmful UV rays of the Sun are absorbed by ozone layer. The ozone layer in the stratosphere prevents the UV rays from reaching the earth surface and thus, protects living organisms, from its harmful effects. That is why ozonosphere is so essential.
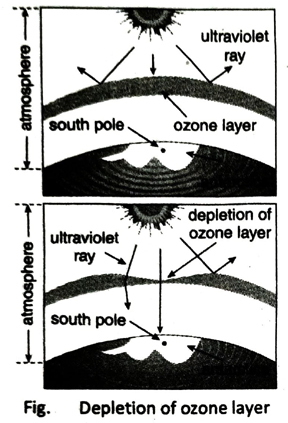
Q.2 What is ozone hole?
Ans. Excessive use of different chemicals and technological devices for the development of human civilization leads to a gradual depletion of the ozone layer. The rate of decomposition of ozone is much higher than the rate of formation of it. As a result hole is formed in certain regions of the ozone layer which is known as ozone hole.
Q.3 Name some man-made chemical substances which are responsible for ozone layer depletion.
Ans. Chlorofluorocarbons (CFCs) and some oxides of nitrogen such as nitric oxide (NO) and nitrogen dioxide (NO2) are mainly responsible for depletion of ozone layer.
Q.4 What is the unit used to measure concentration of ozonosphere? Define the unit.
Ans. The concentration of ozone layer is measured in Dobson unit.
At 0°C temperature and 760 mm of Hg pressure the density of 0.01 mm thick ozone gas layer is called 1 Dobson Unit (1 DU).
Q.5 Describe the significance of ozone layer on the environment.
Ans. Ozone layer in stratosphere acts as a protective shield for the earth. If the ozone layer was not present in the stratosphere, the ultraviolet rays would reach the earth surface. As a result- (1) the overall temperature of the earth surface would increase. The increased temperature would lead to extinction of plants and animals on the earth. (2) direct exposure to ultraviolet rays may cause a number of diseases including skin cancer, premature cataract etc.
Q.6 How is ozone layer formed?
Ans. UV-C (wavelength: 100-280 nm) along with some UV-B (wavelength: 280-315 nm) decomposes oxygen molecules present in the stratosphere into atomic oxygen. These oxygen atoms then combine with molecular oxygen to form ozone (O3) molecules. The last step is highly exothermic in nature, i.e., large amount of heat is produced in this step.
Thus ozone gas is formed in this layer.
Q.7 Discuss the natural causes that decompose the ozone present in ozonosphere. How is the total amount of ozone gas maintained in the ozone layer?
Ans. Dissociation of ozone molecules in the stratosphere occurs simultaneously with its formation. Ultraviolet rays of larger wavelength (UV-A; wavelength: 315-400 nm) decomposes ozone molecules into molecular oxygen and atomic oxygen.
In stratosphere, formation of ozone molecules and decomposition of ozone molecules occur simultaneously in a cyclic manner and a dynamic equilibrium is maintained between these two processes. As a result, the amount of ozone gas in the stratosphere remains constant.
Q.8 Ozone gas present in stratosphere is important but presence of ozone gas in troposphere is harmful-justify the statement.
Ans. The harmful UV rays of the Sun are absorbed by ozone layer present in the stratosphere. Hence, this stratospheric ozone is protecting the earth from UV rays and its harmful effects as a natural umbrella.
On the other hand, ozone gas present in troposphere acts as a greenhouse gas (it contributes about 7-8% to greenhouse effect). Greenhouse effect plays an important role in the gradual increase of the average temperature of the earth.
Q.9 How is the dynamic equilibrium maintained in the stratosphere during the formation and depletion of ozone layer?
Ans. Formation of ozone layer: Oxygen molecules present in the stratosphere absorb UV-rays and are dissociated to form oxygen atoms.
O2 + UV-rays → O + O
This atomic oxygen combines with molecular oxygen to form ozone molecules-
O2 + O → O3
Depletion of ozone layer: Ozone decomposes to oxygen molecules by absorbing UV-rays.
O3 + UV-ray → O2 + O ; O3 + O → 2O2
Formation of ozone molecules and decomposition of ozone molecules-these two opposite processes continue in cycles at the ozone layer and an equilibrium is established. It is for this equilibrium that the amount of ozone gas in the stratosphere remains constant.
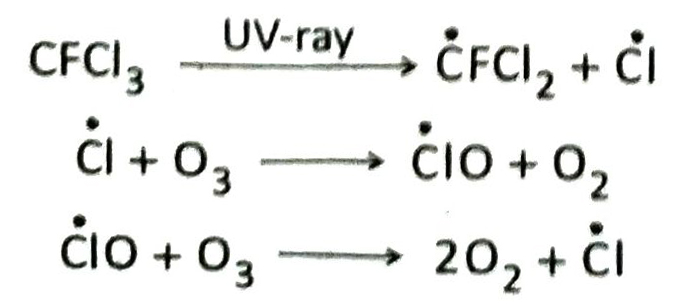
Q.10 Discuss the role of chlorofluorocarbons (freons) in the depletion of ozone layer.
Ans. Chlorofluorocarbons (CFCs) act as catalysts in the depletion of ozone layer in the stratosphere. In presence of ultraviolet rays, CFCs dissociate to form active chlorine atoms (Cl). Active chlorine atom reacts with ozone molecule to form oxygen and chlorine monoxide. Chlorine monoxide further reacts with ozone molecule to form oxygen and active chlorine. This process continues in a cyclic manner. As a result, the density of ozone layer decreases.
Q.11 How are supersonic jet planes responsible for depletion of ozone layer?
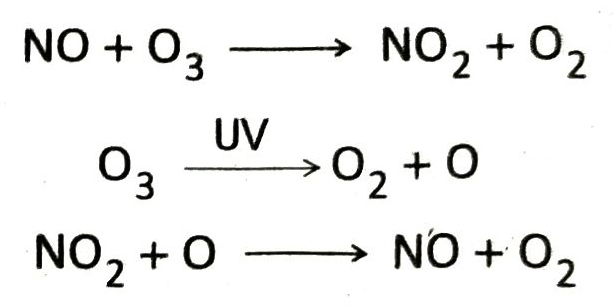
Ans. Supersonic jet planes flying through the stratosphere emits large amount of nitric oxide. Nitric oxide (NO) reacts with ozone to form nitrogen dioxide (NO2) and oxygen. On the other hand in stratosphere, ozone molecules are decomposed by the UV rays to produce atomic oxygen, which reacts with NO2 to produce back NO. Thus, the amount of NO never decreases and causes gradual destruction of ozone molecules. As a result depletion of ozone layer takes place.
Q.12 Discuss the harmful effects of ozone layer depletion.
Ans.
- Ozone layer in the atmosphere absorbs harmful UV rays which would otherwise, reach the earth surface. This would cause an increase in the earth’s temperature resulting in melting of snow-caps in polar regions. Thus the water level of the seas and oceans would increase and flood the coastal regions.
- Exposure to UV rays may cause skin cancer and premature cataract in the eye, natural immunity also decreases. These rays are responsible for formation of photochemical smog.
- UV rays inhibit the process of photosynthesis in plants, hence production of crop gets reduced.
Q.13 What are greenhouse gases?
Ans. Some gases present in the atmosphere absorb radiations of comparatively greater wavelengths (re-radiated from the earth surface) and reflects back the remaining radiations, thus they keep the earth’s atmosphere warm. These gases are known as greenhouse gases.
Some examples of greenhouse gases are carbon dioxide (CO2), methane (CH4), chlorofluorocarbon (CFC), ozone (O3), nitrous oxide (N2O), water vapour etc.
Q.14 What is greenhouse effect?
Ans. Infrared (IR) light coming from the Sun accounts for the heating of earth. As it has a smaller wavelength the gases present in the atmosphere are unable to absorb this incident radiation. The IR light re-radiated from the earth surface is of longer wavelengths. Greenhouse gases like CO2, CH4, CFC, N2O, O3 etc. absorb these re-radiated wavelengths and thus prevent the heat of the Sun to escape from the earth’s atmosphere. As a result the earth’s atmosphere stays warm and thus a favourable environment is created for the survival of living beings. This phenomenon is known as greenhouse effect.
Q.15 Discuss the importance of greenhouse effect.
Ans. Greenhouse gases prevent the reflected radiations to escape from the earth. As a result, the earth’s atmosphere stays warm and thus a suitable environment is created for the survival of living beings. If there were no greenhouse gases in the atmosphere, there would have been no greenhouse effect and the earth’s average temperature would fall to -30°C.
Q.16 How do greenhouse gases increase the temperature of the atmosphere?
Ans. IR rays (of shorter wavelengths) of the Sun can easily penetrate the lining of the greenhouse gases (CO2, CH4, O3, N2O, CFC etc.) present in the atmosphere. The earth’s surface absorbs some part of this radiation and becomes heated. Remaining part of this radiation is retransmitted. IR rays radiated from the heated earth’s surface is of greater wavelengths and got absorbed by the screen of greenhouse gases. Thus the layer of greenhouse gases maintains the warmth of the atmosphere within a certain range by trapping the retransmitted IR rays. But the amount of greenhouse gases in the atmosphere is gradually increasing due to human activities as well as by some natural causes. As a result greater amount of surface radiated IR rays (of longer wavelengths) are getting trapped in the atmosphere which in turn increases the mean temperature of the earth. Thus greenhouse effect influences rather accelerates the phenomenon of global warming.
Q.17 Name the sources of CO2 in atmosphere. Discuss its role in creating greenhouse effect.
Ans. Sources of CO2 in atmosphere: (1) Large quantities of CO2 is added to the atmosphere due to uncontrolled use of fossil fuels in automobiles and factories. (2) In different industries, mainly in case of cement production, large amount of CO2 is produced. (3) Cutting down of trees in indiscriminate manner results in the rise of the amount of CO2 in atmosphere.
Role of CO2 in creating greenhouse effect: Amount of CO2 in atmosphere is maximum, so it has the highest contribution in creating greenhouse effect. Its contribution in greenhouse effect is more than 50%.
Q.18 Name the sources of methane gas. Discuss the role of methane gas in greenhouse effect.
Ans. Sources of methane gas: Methane gas is produced in wetlands due to anaerobic decomposition of dead plants and animals by the action of methanogenic bacteria (methanococcus, methanobacterium). Methane gas is found in oil mines. 3 Decomposition of several organic wastes and the excreta of different animals release methane to the atmosphere.
Role in greenhouse effect: The capability of a molecule of methane to trap the re-radiated heat energy is 25 times greater than that of a molecule of CO2. But the amount of methane in the atmosphere is much lower than that of carbon dioxide. Hence the contribution of methane in greenhouse effect is lower than that of CO2 which is nearly around 19-20%.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. The coldest region of the atmosphere is
A. tropopause
B. stratopause
C. mesopause
D. thermopause
Ans. C
2. Heat reaches the earth surface from the Sun by
A. conduction
B. convection
C. radiation
D. all of these
Ans. C
3. The depletion of ozone layer over the Antarctic region is maximum in the months of
A. March-August
B. April-June
C. September-November
D. January-March
Ans. C
4. Oxides of which element is responsible for the decomposition of ozone molecules?
A. carbon
B. nitrogen
C. hydrogen
D. sulfur
Ans. B
5. CFC dissociates in the presence of ultraviolet rays to produce
A. active carbon atoms
B. active chlorine atoms
C. active fluorine atoms
D. active hydrogen atoms
Ans. B
6. Which oxide of nitrogen emitted from the supersonic jet planes causes depletion of ozone layer?
A. NO2
B. N2O
C. NO
D. N2O4
Ans. C
7. The concentration of ozone in ozonosphere is around
A. 30 ppm
B. 10 ppm
C. 40 ppm
D. 20 ppm
Ans. B
8. Which of the following is a greenhouse gas?
A. O2
B. N2
C. O3
D. H2
Ans. C
9. Which of the following is not a greenhouse gas?
A. CO2
B. NO2
C. CH4
D. N2O
Ans. B
10. The contribution of CO2 towards Greenhouse effect is almost
A. 30%
B. 20%
C. 50%
D. 60%
Ans. C
11. Naturally, ozone gas is produced due to the reaction between
A. CFC and O2
B. UV rays and O2
C. IR rays with O2
D. O2 and water vapour
Ans. B
12. The correct order of contribution of the given gases towards greenhouse effect is
A. CO2 > CH4> CFC > O3
B. CFC > O3> CH4> CO2
C. O3 > CH4> CFC > CO2
D. CH4 > CO2 > O3 > CFC
Ans. A
13. Which would inhibit photosynthesis if it reaches the earth’s surface?
A. infrared radiation
B. ultraviolet radiation
C. radio radiation
D. none of these
Ans. B
14. Source of CFC is-
A. refrigerator
B. vehicles
C. agricultural fields
D. water-bodies
Ans. A
15. Which of the following greenhouse gas has the maximum contribution in increasing the temperature of the earth?
A. N2O
B. CH4
C. CO2
D. H2O vapour
Ans. C
16. Which of the following is required to form ozone layer?
A. ultraviolet ray
B. visible light
C. microwave
D. radiated heatwave
Ans. A
17. Full name of CFC is-
A. chlorofluorocarbon
B. cold fluorinated carbon
C. carbonfluro chloride
D. chlorofluoro carbonate
Ans. A
18. Ozone layer is formed in the atmosphere due to
A. chemical reactions
B. photochemical reactions
C. nuclear reactions
D. electrochemical reactions
Ans. B
Answer in brief
1. Give an example of a gas which is responsible for the gradual increase of atmospheric temperature.
Ans. Carbon dioxide.
2. What percentage of ozone gas found in the atmosphere is present in ozonosphere?
Ans. Ozonosphere contains almost 90% of total ozone gas present in the atmosphere.
3. What happens when ozone molecules absorb ultraviolet rays?
Ans. They decompose to form oxygen molecules.
4. How are UV rays harmful to the eyes?
Ans. Ultraviolet (UV) rays damage the retina of the eyes and may cause premature cataract.
5. Which instrument is used to measure the density of the ozone gas in atmosphere?
Ans. Dobson spectrometer.
6. How many ozone molecules can be destroyed by an active chlorine atom?
Ans. Over 100,000 ozone molecules.
7. What would be the average temperature of the earth if the greenhouse gases were not present in atmosphere?
Ans. In absence of the greenhouse gases, the average temperature of the earth would have been -30°C.
8. What is the average temperature of the atmosphere due to the presence of greenhouse gases?
Ans. Due to the presence of greenhouse gases, the average temperature of the atmosphere is maintained around 15°C.
9. Which diseases may flourish due to global warming?
Ans. Diseases like dengue, malaria, encephalitis may flourish due to global warming.
10. What do you mean by global warming?
Ans. The phenomenon of gradual increase of earth’s temperature due to greenhouse effect is known as global warming.
11. What is the ‘natural sunscreen’ or ‘natural solar screen’ or the ‘umbrella of the earth’?
Ans. The ozonosphere of the stratosphere.
12. Incidence of which ray from the Sun is prevented by the ozone layer?
Ans. UV-ray.
13. Which layer absorbs the Sun’s ultraviolet rays?
Ans. Ozone layer present in the stratosphere.
14. Write down the full form of ODS?
Ans. Ozone Depleting Substances.
15. What is ‘Chapman Cycle’?
Ans. The cycle of creation and destruction of ozone in the stratosphere is called the Chapman Cycle.
16. What is the full form of ODP?
Ans. Ozone Depletion Potential.
17. Which atom of CFC is responsible for the destruction of the ozone layer?
Ans. Cl atom of CFC is responsible for this.
18. Which gas breaks down the ozone layer during lightning?
Ans. Nitric oxide (NO) gas.
19. In which year was the Montreal Protocol signed?
Ans. Montreal Protocol was signed in 1987.
20. Write the name of an organic greenhouse gas.
Ans. Methane (CH4).
21. Which is the main greenhouse gas?
Ans. Carbon dioxide (CO2).
22. Write the full form of GWP.
Ans. Global Warming Potential.
23. Mention a detrimental effect of global warming.
Ans. As global worming increases, polar ice caps will melt and as a result coastal area will be flooded.
Fill in the blanks
1. Ozonosphere is present in the ……….
Ans. stratosphere
2. …………. was the first to observe the formation of holes in the ozone layer.
Ans. Joe Farman
3. The full form of CFC is …………
Ans. chlorofluorocarbon
4. Ozone is a gas with ……….. colour and a ………. smell.
Ans. pale blue, pungent
5. Ozone gas is an allotrope of …………… gas.
Ans. oxygen
6. Density of ozone gas in atmosphere is measured in ………. unit.
Ans. Dobson
7. Nitrous oxide and water vapour are ………… gases.
Ans. greenhouse
8. …………. layer of the atmosphere is also known as the ‘earth’s protective umbrella’.
Ans. Ozone
9. The capacity of a molecule of methane to retain the absorbed heat energy is ………. times more than that of a CO2 molecule.
Ans. 25
10. The wavelength of infrared radiation ………… with decreasing energy.
Ans. increases
11. The rate of increase of CFC gas in the atmosphere per year is ………….
Ans. 5%
12. The gas emitted by supersonic aircraft breaks down the ozone layer is ………..
Ans. nitric oxide
13. The destruction of the ozone layer can cause disease like ……….
Ans. skin cancer
14. An inorganic greenhouse gas is …………
Ans. carbon dioxide
15. The ………… present in the body spray is a greenhouse gas.
Ans. chlorofluorocarbon
16. The greenhouse effect is causing …………
Ans. global warming
State whether true or false
1. Nitrogen is a greenhouse gas.
Ans. False
2. Supersonic jets release huge amount of nitric oxide.
Ans. True
3. Scientist Joe Farman first noticed the formation of ozone hole over Antarctica.
Ans. True
4. Under the influence of UV-rays, CFC splits and produces methane gas.
Ans. False
5. The Montreal Protocol was signed in the year 1987.
Ans. True
6. Damage to the ozone layer will increase the chance of cataracts.
Ans. True
7. It would have been easier for the fauna to survive if there were no greenhouse gases in the atmosphere.
Ans. False
8. The two gases responsible for the greenhouse effect are nitrogen and oxygen.
Ans. False
9. Among all greenhouse gases the contribution of carbon dioxide is maximum in creating greenhouse effect.
Ans. True
10. If there were no greenhouse gases, the temperature of the earth would be 50°C.
Ans. False
TOPIC – C
Proper Uses of Energy and Sustainable Development
SHORT AND LONG ANSWER TYPE QUESTIONS
Q.1 What is sustainable development?
Ans. The controlled and systematic use of natural resources to meet the need of present generation as well as sustaining resources for the future generation is called sustainable development.
Q.2 Discuss the purposes of ‘sustainable development’.
Ans. Purposes of sustainable development are
- Social welfare: Major goal of sustainable development is to establish social equality and eradicate the differences that arise from social misconceptions, superstitions, backward traditions etc.
- Economic development: No society can prosper without economic development. It is required in the field of education, health, agriculture and industries. Hence, one of the major goals of sustainable development is to achieve economic development.
- Ecological development: Ecology of earth depends on the constant interaction between living and non-living things. Sustainable development also aims at maintaining proper balance in nature and building a stronger relationship between living & non-living world.
Q.3 Why is sustainable development considered to be of utmost importance for present as well as future generation?
Ans. (1) The aim of sustainable development is to use natural resources without affecting the environment. This will enable both present and future generation to preserve the natural resources and utilize them for a long time. (2) It gives importance not only to economic development but also to cultural, social & geopolitical development. (3) Sustainable development have been able to control the declination of environmental conditions as it encourages to decrease the use of conventional sources of energy and increase the use of alternative resources.
Q.4 What are fossil fuels?
Ans. Fossil fuels are the natural fuels formed due to the action of geothermal heat, pressure and bacterial degradation of the dead plants and animals buried beneath the earth’s crust for many years. Coal, petroleum, natural gas are examples of fossil fuels. These are exhaustible and nonrenewable sources of energy.
Q.5 What do you mean by conventional or le sources of energy? non-renewable sources of energy? Give examples.
Ans. The sources of energy which are gradually exhausting due to their prolonged uses and which cannot be renewed are called conventional or non-renewable sources of energy. These have been used as the major energy source till date and hence their reserves are gradually running out. Coal, petroleum, natural gases are examples of conventional or non-renewable sources of energy.
Q.6 What are non-conventional or renewable sources of energy? Give examples.
Ans. The sources of energy that have presently been used as alternative energy sources to minimize the uses of fossil fuels and which are inexhaustible and renewable are known as nonconventional or renewable sources of energy.
Solar energy, wind energy, biomass energy, tidal energy etc. are examples of non-conventional or renewable sources of energy.
Q.7 How is electricity generated from wind rated from energy?
Ans. Practically, the kinetic energy of the wind is harnessed to obtain wind energy. Using windmills, the wind energy can be converted into electrical energy. The blades of the windmills are connected to the turbines of an electrical generator. When the blades rotate by the action of wind, the turbines also rotate and electricity is generated.
Q.8 How is electricity generated using tidal energy?
Ans. During high tides, the surging water is stored in a dam and during the low tides this stored water is released, which comes down with a high velocity. Turbines of electrical generators are placed in the path of moving water and thereby the kinetic energy of water can be converted into electrical energy.
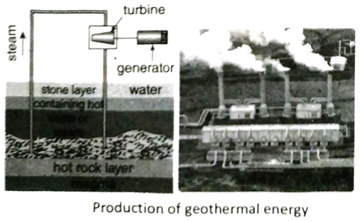
Q.9 How is electricity generated using geothermal energy?
Ans. During volcanic eruptions most of the magma coming out from the earth, gets stuck in the rocks near the earth surface at a depth of 5 to 10 km. These rocks, in contact with the magma gets heated up. When water passes through these hot rocks, it vaporizes to form steam. The steam is piped out directly from the underground wells to the power plants. The kinetic energy of the steam rotates the turbines and generates electricity.
Q.10 What are methanogenic bacteria? Give examples.
Ans. Methanogenic bacteria can be defined as the microorganisms that produce methane gas by the anaerobic decomposition of biomass.
Some examples of methanogenic bacteria are Methanococcus, Methanobacterium etc.
Q.11 Discuss the role of methanogenic bacteria in increasing the amount of methane in atmosphere.
Ans. Methanogenic bacteria decompose dead plants in wetlands, rain-forests etc. to produce methane gas. Thus, the amount of methane in atmosphere increases.
Q.12 What is biomass energy? What are the disadvantages of using biomass energy?
Ans. Biomass refers to the dead plants and plant remains, domestic wastes, agricultural wastes,. animal excreta, animal carcasses etc. Energy stored in biomass is known as biomass energy.
Biomass energy is considered to be one of the major alternative sources of energy to replace fossil fuels. However, it has some harmful effects on environment as the combustion of biomass causes air pollution.
Q.13 What is biogas? What are its constituents?
Ans. Biomass refers to dead plants and plant remains, domestic wastes, agricultural wastes, animal excreta, animal carcasses etc. The biomass is enclosed in a large chamber and decomposed by the action of methanogenic bacteria to produce a combustible gas, known as biogas.
The major components of biogas is methane (CH4). Apart from methane, carbon dioxide (CO2) and traces of H2, N2, CO, O2, H2S and water vapour are also present in it.
Q.14 Write some uses of biogas.
Ans. (1) Biogas can be used as a fuel to light lamps, to heat water and for cooking purpose.
(2) In many countries, it is used to dry bricks, ceramic tiles, tobacco etc.
(3) Electricity generated from biogas can be used to run small pumps and small scale industries.
Q.15 What is biofuel? Give examples.
Ans. The fuel produced from biomass is known as biofuel.
Bioethanol produced by fermentation of corn or sugar cane is an example of biofuel. It is mixed with petrol and used as automobile fuel.
Q.16 What is methane hydrate?
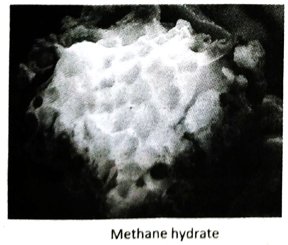
Ans. Methane hydrate is a crystalline solid substance (clathrate compound) in which a large amount of methane molecules are trapped inside the cagelike lattice of ice. It is represented by the formula 4CH4 · 23H2O. It exists only under suitable temperature and pressure.
Q.17 How is methane present in coal mines?
Ans. Due to bacterial action or geothermal heating, methane gas is produced in coal seams. The coal seams are saturated by ground water and the produced water pressure causes methane gas to get adsorbed in the coal seam.
Q.18 Why is methane gas extracted before the mining of coal?
Ans. Before the mining of coal, methane gas should be extracted as much as possible from the coal bed. This is because- (1) it will reduce the probability of catching fire and chances of accidents can be averted, (2) the extracted methane gas can be used as a potential fuel.
Q.19 Why is methane hydrate considered as an important source of energy?
Ans. Recent discovery of methane hydrate explores a new dimension of energy resources. Scientists have estimated that methane hydrate contains more carbon than all the fossil fuel available on the earth, combined together. 1Litre of methane hydrate is found to contain 170 Litre of methane gas in STP. When the world is facing an energy crisis due to exhaustion of fossil fuels, methane hydrate provides a complete new direction towards energy resources.
Q.20 State the concerns of using methane hydrate as a potential source of energy.
Ans. (1) Methane hydrate is not as stable as other minerals. Due to increase in temperature or decrease in pressure, it may be transformed into water and methane gas may be released from the mineral. This may cause landslide beneath the earth surface. (2) Methane is a major greenhouse gas. Heating methane hydrate may release methane gas to the atmosphere which may lead to global warming.
Q.21 Discuss some advantages of using solar energy.
Ans. (1) Solar energy is inexhaustible and renewable. Scientists consider it as the most important source of alternative energy. (2) Unlike fossil fuels, solar energy does not cause pollution. (3) This source of energy is very effective in less populated areas and in those regions where the conventional sources of energy are not widely available. (4) The production of solar energy requires minimum natural resources.
Q.22 Mention some disadvantages of using solar energy.
Ans. (1) Production cost of solar energy is very high as it requires complex technological support. So, under-developed countries find this energy unsuitable for use. (2) Initial cost of installing solar panel is very high. This is one of the major disadvantages of solar energy. (3) Areas which do not get sufficient sunlight, are not suitable for utilising solar energy. (4) Solar energy centres produce solar energy in limited quantity which can satisfy only the local need.
Q.23 Mention some advantages of wind energy.
Ans. (1) Wind energy is renewable and is source is inexhaustible. Even it’s incessant use will not create energy crisis. (2) Even though initial cost of installation is somewhat high, regular cost is very low as only maintenance cost is required. (3) Wind energy does not cause pollution. (4) It requires minimum technological support.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. Which of the following is not a fossil fuel?
A. coal
B. petrol
C. solar energy
D. diesel
Ans. C
2. Among petrol, diesel, kerosene and LPG, which one has the highest calorific value?
A. petrol
B. kerosene
C. diesel
D. LPG
Ans. D
3. Which of the following represents the correct order of calorific values of the corresponding fuels?
A. coal < diesel < hydrogen < LPG
B. coal < hydrogen < diesel < LPG
C. coal < diesel LPG < hydrogen
D. coal LPG < diesel < hydrogen
Ans. C
4. Which of the following is a fossil fuel?
A. coal
B. petroleum
C. natural gas
D. all of these
Ans. D
5. Fossil fuels need to be conserved because
A. these are very expensive
B. these are not easily available
C. these are non-renewable
D. none of these
Ans. C
6. Which of the following elements is the major constituent of solar cells?
A. Li
B. Na
C. Si
D. Cu
Ans. C
7. The major source of energy which meets our daily demands is
A. solar energy
B. wind energy
C. tidal energy
D. fossil fuels
Ans. D
8. Solar energy gets converted into electrical energy in a/an
A. photo voltaic cell
B. solar cooker
C. pressure cooker
D. electric motor
Ans. A
9. The least polluting fossil fuel is
A. diesel
B. coal
C. kerosene
D. natural gas
Ans. D
10. A source of non-renewable energy is
A. wind energy
B. petroleum
C. tidal energy
D. solar energy
Ans. B
11. The energy stored in coal, petroleum etc. is
A. wind energy
B. solar energy
C. tidal energy
D. renewable energy
Ans. B
12. Which gas found in coal mines is used as a fuel?
A. ozone
B. methane
C. oxygen
D. nitrogen
Ans. B
13. An example of methanogenic bacteria is
A. Methanococcus
B. Methanobacterium
C. both A and B
D. none of these
Ans. C
14. Which of the following does work on the principle of greenhouse effect?
A. solar cooker
B. radio station
C. windmill
D. biogas plant
Ans. A
15. The largest windmill of India is situated in
A. Kanyakumari (approx. 380MW)
B. Surat (approx. 220MW)
C. Haridwar (approx. 460MW)
D. Sagardwip (approx. 410MW)
Ans. A
16. The state with the largest installed wind power generation capacity in India is
A. Tamil Nadu
B. West Bengal
C. Kerala
D. Maharashtra
Ans. A
17. Which of the following are eco-friendly fuels-(i) coal, (ii) kerosene, (iii) natural gas, (iv) biogas?
A. (i) and (iv)
B. (iii) and (iv)
C. (i) and ii
D. (i) and (iii)
Ans. B
18. Which type of coal has the highest calorific value?
A. Pit
B. Lignite
C. Anthracite
D. Bituminous
Ans. C
19. The main component of biofuel is-
A. carbon dioxide
B. ethanol
C. methanol
D. ether
Ans. B
20. Solar cells are formed by
A. conductors
B. insulators
C. semiconductors
D. super conductors
Ans. C
21. Source of the solar energy is
A. nuclear fission
B. coal
C. nuclear fusion
Ans. C
22. For which ray of sunlight does a solar cooker work?
A. Gamma ray
B. Infrared ray
C. Ultraviolet ray
D. Visible ray
Ans. B
Answer in brief
1. What do you mean by alternative fuels?
Ans. Alternative fuels are those which are being used to restrict the use of fossil fuels in order to conserve their sources as these are gradually running out.
Example: solar energy, wind energy etc.
2. Mention two major sources of energy.
Ans. (1) Conventional sources of energy and
(2) Non-conventional sources energy.
3. Name a conventional and renewable source of energy.
Ans. Wind power.
4. Write the name of a conventional and nonrenewable source of energy.
Ans. Coal (fossil fuel).
5. Write the names of two non-conventional and renewable sources of energy.
Ans. (1) Solar energy, (2) Tidal energy.
6. Write the names of two non-conventional and non-renewable sources of energy.
Ans. (1) Geothermal energy, (2) Nuclear energy.
7. Why is the inner surface of a solar heater painted black?
Ans. Black surface can absorb more heat from sunlight. So, the inner surface of a solar heater is painted black.
8. Which fuel has the highest calorific value?
Ans. Hydrogen (150000 kJ/kg) has the highest calorific value.
9. Why do the pipelines of natural gas often get blocked?
Ans. The pipelines of natural gas often get blocked due to the formation of solid methane hydrate.
10. The gas trapped in coal mines is a potential source of energy. Name the gas. Why is it called firedamp?
OR, What is firedamp?
Ans. The gas is methane (CH4).
Methane gas accumulated in coal-mines forms an explosive mixture when mixed with air and often causes fire in coal mines. So this gas is named as firedamp.
11. How is electricity produced in solar cells?
Ans. When photons present in sunlight are incident on the surface of the solar cell (made up of semiconductors, such as, Si), the electrons get excited and ultimately becomes free. These free electrons generate electricity.
12. What is geothermal energy?
Ans. When groundwater passes over hot beds of rocks, it gets vaporised. These vapours are utilized to move the plates of a turbine thereby producing electrical energy. This is known as geothermal energy.
13. What is tidal energy?
Ans. During tides, the kinetic energy of water can be converted into electrical energy using turbines. This energy is known as tidal power or tidal energy.
14. Which is the major raw material used for the production of biogas?
Ans. Cow dung.
15. What is calorific value of a fuel?
Ans. The amount of heat produced due to the complete combustion of 1 kg of a fuel is known as its calorific value. Its value is represented in kJ . kg-¹ unit in Sl.
16. How is biodiesel produced?
Ans. Biodiesel is produced due to the transesterification of vegetable oil and animal fats.
17. Which seeds are used to produce biodiesel in India?
Ans. The seeds of jatropha tree are used to produce biodiesel in India.
18. What is ‘methane hydrate’?
Ans. Methane hydrate is a crystalline solid where methane molecules remain trapped inside a cage-like lattice of ice.
19. Write down the unit of calorific value of a fuel?
Ans. The SI unit of calorific value of a fuel is J/kg and the CGS unit is cal/g.
20. Calorific value of LPG is 50 kJ/g -Explain.
Ans. It means that 50 kJ of heat is obtained by combustion of 1 g of LPG.
21. Arrange in ascending order of calorific value-Kerosene, Wood, Coal, Hydrogen, LPG, Diesel.
Ans. Wood Coal > Diesel > Kerosene > LPG > Hydrogen
22. Write the name of a fossil fuel.
Ans. Coal
23. Which gas is filled in the gas cylinder used for cooking?
Ans. Liquefied Petroleum Gas (LPG).
24. What is the main component of LPG?
Ans. Butane.
25. What is the main component of CNG?
Ans. Methane.
26. Which of the following is a fossil fuel? Charcoal, Petrol, Ethanol.
Ans. Petrol.
27. Which gas is produced during the combustion of fossil fuels?
Ans. CO2 (Carbon dioxide).
28. Write the name of a conventional renewable pollution free energy source.
Ans. Wind power.
29. Write the name of an environment friendly non-conventional energy source.
Ans. Geothermal energy.
30. Write the name of an alternative energy source.
Ans. Solar energy.
31. Write the name of a device that uses solar cells.
Ans. Solar calculator.
32. What is generated from the wind power of wind mill?
Ans. Electrical energy.
33. Write the name of a place in West Bengal that has wind mills.
Ans. Fraserganj.
34. Name an energy source that does not come directly or indirectly from the sun.
Ans. Geothermal energy.
35. What is available as residue in controlled construction of wood?
Ans. Charcoal.
36. Methanogenic Bacteria decompose which substance to produce methane gas?
Ans. Biomass.
37. Give an example of Methanogenic Bacteria.
Ans. Methanogens-Methanosarcina barkeri
38. Mention an use of Biogas.
Ans. Biogas can be used as a fuel for cooking purpose.
39. Write down the fuel form of CBM.
Ans. Coalbed methane.
40. What is sweet gas?
Ans. Methane gas obtained from coal-mines is called sweet gas.
41. What is ‘fiery ice’?
Ans. Methane Hydrate ((4CH4 · 23H2O) is called ‘fiery ice’. .
42. How much volume of methane gas can be obtained from 1 Litre of methane hydrate at NTP?
Ans. Almost 170 Litre of methane gas can be obtained from 1 Litre of methane hydrate at NTP.
Fill in the blanks
1. The calorific value of LPG is …………
Ans. 55000 kJ/kg
2. The major component of biogas is ………..
Ans. methane
3. Apart from methane, another gas that is found in biogas is ………..
Ans. CO2
4. At STP, about ………… methane is obtained from 1 L of methane hydrate.
Ans. 170 L
5. Methane hydrate is solid ………….compound.
Ans. clathrate
6. ………… river basin in India has one of the largest reserves of methane hydrate.
Ans. Krishna-Godavari
7. Idea of sustainable development is properly developed in the report of ………… Commission.
Ans. Brundtland
8. Biogas is produced due to anaerobic decomposition of cow dung by ……….. bacteria.
Ans. methanogenic
9. The calorific value of methane is …………. than petrol.
Ans. less
10. In photo-voltaic cells, solar energy is directly converted into ………. energy.
Ans. electrical
11. In artificial satellites, the main source of energy is the energy produced by …………
Ans. solar cells
12. The value of solar constant is approximately ……………
Ans. 1.4 kW/m²
13. Biogas contains …………. % of methane gas.
Ans. 50 – 80
14. Among wood and methane the calorific value is more in case of ………….
Ans. methane
15. The substance used to make solar cell is …………
Ans. silicon
16. Electrical energy is obtained from …………… in solar cell.
Ans. solar energy
17. ………….. is generated from wind power in wind mills.
Ans. Mechanical energy
18. …………… in West Bengal is a region which is rich in geothermal energy,.
Ans. Bakreshwar
19. Vegetable wastes are decomposed by anaerobic bacteria in …………. of air in the biogas plants.
Ans. absence
20. To produce biogas ……………. bacteria is needed.
Ans. methanogenic
21. The hard coal in the mine contains a large amount of ………….. in the absorbed state.
Ans. methane
22. …………… is also termed as fiery ice.
Ans. Methane hydrate
23. …………… gas is obtained from methane hydrate.
Ans. Methane
State whether true or false
1. The calorific value of kerosene is greater than LPG.
Ans. False
2. Semiconductors like Si, Ge etc., are used in solar cells.
Ans. True
3. Gasoline is a kind of biofuel.
Ans. False
4. Methanogenic bacteria play a major role in biogas production.
Ans. True
5. CO2 gas is trapped inside the coal mines.
Ans. False
6. Quality of fuel is determined by its calorific value.
Ans. True
7. Methane hydrate contains 13.3% methanë.
Ans. False
8. Bioethanol is a liquid biofuel.
Ans. True
9. The source of solar energy is the nuclear fission reaction that takes place inside the Sun.
Ans. False
10. The chemical energy stored in biomass is called the biomass energy.
Ans. True
11. Solar panels are used in broadcast relay stations.
Ans. True
12. The most widely used definition of sustainable development was produced by the report of the Brundtland Commission.
Ans. True
13. Windmills usually convert electrical energy into mechanical energy.
Ans. False