WBBSE 9th Class Science Solutions Physical Science & Environment Chapter – 4.1 Atomic Structure
West Bengal Board 9th Class Science Solutions Physical Science & Environment Chapter – 4.1 Atomic Structure
WBBSE 9th Class Physical Science & Environment Solutions
Synopsis
- Subatomic particles of an atom: Electron, proton and neutron are the three main subatomic particles of an atom.
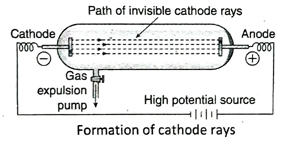
- Cathode rays: A strong potential difference (10000 V) is applied between two electrodes present inside an electric discharge tube containing a gas at a very low pressure (0.01 mm Hg). As a result, the cathode emits certain invisible ray, known as cathode ray which produces a faint greenish fluorescence on the glass wall opposite to that of the cathode.
- Discovery of electrons: Considering various properties of cathode rays, J. J. Thomson concluded (in 1897) that cathode rays are composed of negatively charged material particles and named them negatrons. Later these particles were named electrons.
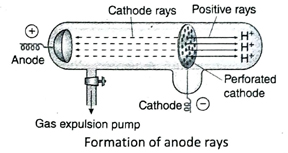
- Anode rays: When perforated cathode is used inside the discharge tube, a positively charged ray is emitted from the anode which moves towards the cathode. This is known as anode ray.
- Discovery of protons: When hydrogen gas was taken in the discharge tube, a positively charged particle was obtained during the anode ray experiment. This particle is known as proton.
TOPIC – A
Concept of Atom
SHORT AND LONG ANSWER TYPE QUESTIONS
1. How is cathode ray produced in an ay produced in an electric discharge tube?
Ans. When gas inside the discharge tube is kept at very low pressure (about 0.01 mm Hg pressure) and a very high potential difference (10000 V) is applied between two metallic electrodes of the discharge tube, an invisible ray is produced at the cathode which then moves towards the anode. This ray is called the cathode ray. It creates fluorescence on the glass wall opposite to that of the cathode in the discharge tube.
2. Why is the charge of an electron considered to be the smallest unit of electricity?
Ans. Scientist Millikan was the first to determine the charge of an electron as -1.602 × 10-19 coulomb or -4.8 × 10-10 esu. However, no negatively charged particle with a lower charge value than an electron has been discovered till date. Thus, the charge of an electron is considered to be the smallest unit of electricity. The magnitude of charge of any positively or negatively charged particle is thus considered to be equal to or an integral multiple of the charge of an electron.
3. How is anode ray produced in an electric e ray produced in an electric discharge tube?
Ans. When gas inside the discharge tube is kept at a very low pressure (about 0.01 mm Hg pressure) and a very high potential difference (10000 V) is applied between two metallic electrodes and if during this process a perforated cathode is used, an invisible ray is produced at the anode which then moves towards the cathode. This ray is called the anode ray. In this condition, if the anode rays are allowed to pass through the perforated cathode, they produce a reddish glow on the glass wall behind the cathode.
4. What is canal ray? Why is it named so?
Ans. Inside the discharge tube, if a high potential difference is applied between the two electrodes at a very low pressure (about 0.01 mm Hg pressure), an invisible ray consisting of positively charged particles is produced at the anode, moves towards the cathode and passes through the perforated cathode in a straight line. This ray is called the canal ray or anode ray.
The ray is so named because it passes through the perforated cathode.
5. Which observation of the Thomson’s discharge tube experiment led to the conclusion that cathode ray is not an electromagnetic radiation?
Ans. In Thomson’s experiment, it was observed that the cathode rays were deflected in the positive direction when an electric field was applied. This proved that cathode ray is not an electromagnetic radiation but a stream of negatively charged particles. If a light paddle wheel is placed in the path of cathode rays, it is seen that the wheel starts rotating. This too proves that cathode ray is a stream of particles having fixed mass.
6. Write four applications of electric discharge through a gas at low pressure.
Ans. Electric discharge through a gas at low pressure has the following applications in- (1) neon-sign advertising boards, (2) television tubes, (3) fluorescent tube, (4) sodium vapour lamp.
7. State state three in important characteristics of cathode rays.
Ans. Three important characteristics of cathode rays are as follows- (1) Cathode rays move in a straight line. If an opaque substance is placed in the path of cathode rays, its shadow is formed. The path of cathode rays remains unaffected by the position of the anode. (2) Cathode ray is a stream of particles, each having a definite but small mass. So, if a light paddle wheel is placed in the path of the cathode rays, it rotates due to collision with the particles. (3) Cathode rays consist of negatively charged particles. It gets deflected in presence of magnetic and electric field. In an electric field, cathode rays deflect towards the positive plate while in a magnetic field, cathode rays deflect towards the north pole.
8. The e/m (charge/mass) ratio of cathode ray is the same for all gases taken in an electric discharge tube, but the e/m ratio of anode ray is different for different gases. Why?
Ans. Cathode ray is a stream of electrons. These electrons are emitted from the metallic cathode when high potential difference is applied between the electrodes in an electric discharge tube containing gases at a very low pressure. Electron is a fundamental particle present in the atoms of all elements. Hence, e/m ratio of cathode ray is constant irrespective of the gas being used in the discharge tube.
On the other hand, anode rays are produced when high potential difference is applied in a discharge tube at a very low pressure. Under these circumstances, the gaseous atoms lose electrons and get converted into cations. Anode ray is the stream of these cations. As the atomic mass is different for different gases, the mass of anode ray particles varies when different gases are used in the discharge tube. Hence, the e/m ratio is different for different gases.
9. How was the existence of protons detected in anode rays?
Ans. The nature of deflection of anode rays in presence of electric and magnetic fields indicate that it is a collection of positively charged particles (it deviates towards the negatively charged plate). Again, it is observed by using different gases in the electric discharge tube that e/m ratio of the anode ray is different for different gases. When hydrogen gas is used, the mass of the positively charged particle obtained in the electric discharge tube is found to be the minimum and the charge of each particle is equal to that of an electron. From this, it was concluded that the particles present in anode ray in the presence of hydrogen gas are similar to H+ ion. This led to the discovery of proton in anode rays by using naturally occurring hydrogen gas.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. The constituent particle of cathode rays is
A. electron
B. proton
C. neutron
D. ion
Ans. A
2. Which of the following is not a fundamental particle of matter?
A. electron
B. proton
C. positron
D. neutron
Ans. C
3. Negatron is also known as
A. electron
B. proton
C. neutron
D. positron
Ans. A
4. The heaviest particle of an atom is
A. neutron
B. proton
C. electron
D. none of these
Ans. A
5. Speed of anode rays
A. is higher than that of cathode rays
B. is much less than that of cathode rays
C. is equal to that of cathode rays
D. cannot be determined
Ans. B
6. Canal ray is commonly known as
A. cathode ray
B. anode ray
C. X-ray
D. UV-ray
Ans. B
7. Cathode ray consists of
A. electron
B. proton
C. neutron
D. ion
Ans. A
8. Which one is not an electromagnetic radiation?
A. cathode ray
B. X-ray
C. radio wave
D. UV-ray
Ans. A
9. Thomson’s atomic model is known as
A. plum pudding model
B. proton-electron model
C. plum cake model
D. pomegranate model
Ans. A
10. Cathode ray is deflected by electric field as well as by magnetic field as it is
A. electromagnetic radiation
B. stream of positively charged particles
C. stream of neutral particles
D. stream of negatively charged particles
Ans. D
11. Nature of charge of an electron is
A. neutral
B. negative
C. positive
D. none of the above
Ans. B
12. A proton is
A. 1836 times heavier than an electron
B. 1746 times heavier than an electron
C. lighter than an electron
D. 1236 times heavier than an electron
Ans. A
13. The particle formed when an electron is removed from a hydrogen atom
A. proton
B. neutron
C. α-particle
D. β-particle
Ans. A
Answer in brief
1. At what pressure are cathode rays produced in an electric discharge tube?
Ans. In an electric discharge tube, cathode rays are produced at a very low pressure of about 0.01 mm of Hg.
2. In which direction will the cathode rays deflect in presence of an electric field?
Ans. In presence of an electric field, the cathode rays will be deflected towards the positive plate.
3. Who found a method to determine the number of protons in an atom?
Ans. Scientist Moseley (in 1913).
4. Why is cathode ray formed at very low pressure only?
Ans. At a very low pressure the resistance inside the discharge tube is less, hence the formation of cathode ray becomes feasible.
5. What happens when cathode rays strike the surface of various hard metals?
Ans. Cathode rays produce X-rays when they strike the surface of various hard metals like tungsten, molybdenum etc.
6. Write down the similarity between cathode rays and ordinary light.
Ans. Just like ordinary light, cathode rays also affect photographic plates.
7. Who discovered electrons?
Ans. J.J. Thomson, in 1897, discovered electrons.
8. Who named the negatively charged particles, present in cathode rays, electrons?
Ans. Scientist G.J. Stoney.
9. Which scientist is considered as the ‘father of atomic physics’?
Ans. John Dalton.
10. Who determined the charge of an electron by oil drop experiment?
Ans. Scientist Robert A. Millikan.
11. Who performed the discharge tube experiment using a perforated cathode?
Ans. Scientist E. Goldstein.
12. Who discovered protons?
Ans. Scientist Ernest Rutherford (in 1911).
13. Who named the positively charged particles, found in anode rays, protons?
Ans. Scientist E. Rutherford.
14. Who proposed the watermelon model or plum pudding model for an atom?
Ans. Scientist J. J. Thomson.
Fill in the blanks
1. Cathode ray is a stream of ……………..
Ans. electrons
2. Positive ray is commonly known as ………. ray.
Ans. anode
3. The nucleus of an atom does not consist of ……………
Ans. electrons
4. Cathode rays are emitted ………… from the cathode surface and travel towards the ………..
Ans. perpendicularly, anode
5. The watermelon model for the structure of an atom was proposed by ………..
Ans. J. J. Thomson
6. ……….. is produced when cathode rays hit the walls of a discharge tube or fall on the surface of substances like ………..
Ans. Fluorescence, zinc sulphide
7. When cathode rays hit the surface of metals like tungsten or molybdenum, ……….. -rays are produced.
Ans. X
8. During production of cathode rays, the pressure in the discharge tube is equal to the pressure of ………. mm mercury column.
Ans. 0.01
State whether true or false
1. Mass of a hydrogen atom is almost same to that of a proton.
Ans. True
2. Cathode ray is streamed from anode to cathode.
Ans. False
3. First discovered subatomic particle is proton.
Ans. False
4. Cathode ray is emitted perpendicularly from the cathode plane.
5. Watermelon model of an atom is also known as the plum pudding model.
Ans. True
6. An electron is 1836 times heavier than a proton.
Ans. False
7. The e/m ratio of cathode ray depends upon the gas used in the discharge tube.
Ans. False
8. The number of protons present influences the mass of an atom.
Ans. True
9. Thomson named anode ray as positive ray.
Ans. True
10. The plum pudding model of Thomson gives the concept of nucleus in an atom.
Ans. False
TOPIC – B
Rutherford’s Atomic Model & Discovery of Neutron
SHORT AND LONG ANSWER TYPE QUESTIONS
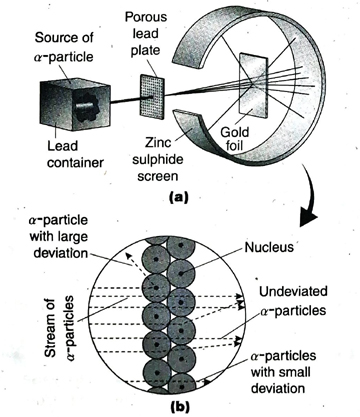
1. On the basis of Rutherford’s α -scattering experiment, answer the given questions- (1) What is an α -particle? (2) Why was gold foil used in α -scattering experiment? (3) What arrangement was made to detect the path of α -particles?
Ans. (1) An α -particle is a particle having a mass of 4 units and 2 units of positive charge. It consists of 2 protons and 2 neutrons. As these are identical with helium nuclei, α-particles are often represented as 24He2+. During disintegration of the nuclei of many radioisotopes, emission of α -particles take place.
(2) Thinner the metal foil used, higher will be the probability of α -particles to collide with the atoms of the metal foil. As gold is a soft and highly malleable metal, it can be easily converted into a very thin foil. Due to this Rutherford used gold foil in his experiment.
(3) A circular fluorescent screen coated with zinc sulphide (ZnS) was set up around the metal foil. After penetrating through the metal foil, the α-particles hit the screen generating a flash of light. This helped in detecting the path of scattered a -particles.
2. State the observations made by Rutherford in his α-scattering experiment.
Ans. Rutherford made the following observations in his a-scattering experiment- (1) Most of the aparticles passed straight through the gold foil and hit the zinc sulphide (ZnS) screen without suffering any deviation. (2) A few α-particles passed through the foil after suffering small deviations. (3) Very few α-particles (1 out of 20000) suffered deviation through large angles or even deflected by an angle of 180°.
3. What were the conclusions drawn by Rutherford from his α-scattering experiment?
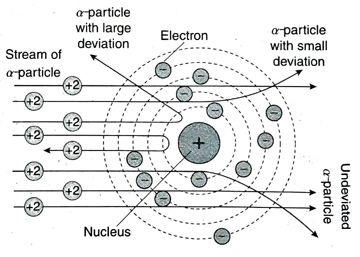
Ans. From the observations of his a-scattering experiment, Rutherford drew the following conclusions- (1) As metal foil is made up of metal atoms and most of the α-particles pass through the gold foil without any deviation, major part of an atom is empty. (2) α -particles are positively charged. So, they can be deflected only by another positive charge having greater mass than themselves. Hence, it was concluded that all the atom is positive charge and mass of an concentrated within a very small region in the atom. This is the nucleus of an atom. (3) Only those α-particles which hit the nucleus suffer deviation by large angles (90° or more) even deflected back at an angle of 180°. (4) Negatively charged electrons remain outside the nucleus. As α-particles are much heavier than electrons, their paths are not affected by the presence of electrons in an atom.
4. Describe Rutherford’s atomic model.
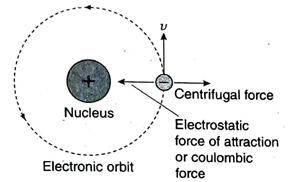
Ans. Based on the conclusions of his α-scattering experiment, in 1911, Rutherford proposed an atomic model known as Rutherford’s atomic model. The postulates of this model are as follows- (1) An atom has two parts-a positively charged nucleus and the extra nuclear part where the negatively charged electrons are present. (2) Entire mass and positive charge of an atom is concentrated within a very small region in the atom. This is known as the nucleus. Most of the space in an atom is empty. (3) The nucleus is very small in size with respect to an atom. The diameter of an atom is around 10-8 cm while that of a nucleus is 10-13 cm. (4) The electrons revolve around the nucleus in different circular orbits. (5) Total number of electrons revolving around the nucleus is equal to the number of positively charged protons present within the nucleus. Two opposite forces act on an electron moving along an orbit-the electrostatic force of attraction between negatively charged electrons and positively charged nucleus and the centrifugal force acting on the electrons due to its rotational motion which acts away from the nucleus. These two forces being equal in magnitude but opposite in direction, maintain the stability of the electrons in the orbit.
5. What is radioactivity? How did the discovery of radioactivity help Rutherford in proposing his atomic model?
Ans. Radioactivity is a nuclear phenomenon in which the nuclei of heavier elements, like uranium, thorium etc., in elemental state or in compound form, disintegrate spontaneously to form nuclei with greater stability (i.e., new elements are formed) and invisible rays are emitted (radioactive radiation) during this process.
From the results of electric discharge through gases at low pressure and the discovery of atom it was evident that atoms can be divided into smaller particles. After the discovery of radioactivity, Rutherford performed his famous α-scattering experiment on the basis of which he proposed his atomic model.
6. Mention the merits of Rutherford’s atomic model.
Ans. Merits of Rutherford’s atomic model are – (1) Existence of nucleus is firstly observed in this model. The concept that almost the entire mass of an atom is concentrated at its nucleus is accepted in future days. (2) The concept of revolving of electrons in several orbits around the nucleus firstly known from this atomic model. (3) Total negative (-ve) charge of revolving electrons is numerically equal to the total positive (+ ve) charge of the nucleus-from this concept, neutrality of an atom can be justified.
7. Why is an atom electrically neutral?
Ans. An atom contains equal number of protons and electrons. Now, the charge of a proton is equal in magnitude but opposite in nature with respect to the charge of an electron. Hence, the total positive charge in the nucleus of an atom is equal to the total negative charge of electrons revolving in the orbits. Hence, an atom is electrically neutral.
8. The electrons outside the nucleus are negatively charged and the protons in the nucleus are positively charged. Then why do the electrons not fall into the nucleus due to its attraction?
Ans. According to Rutherford’s atomic model, the electrons revolve around the nucleus in different circular orbits with high velocity. While rotating in an orbit, the centrifugal force acting on an electron is equal in magnitude to the electrostatic force of attraction acting between the negatively charged electron and the positively charged nucleus. However, these two forces act in opposite directions. Hence, the resultant force acting on an electron is zero and the electron maintains its stability in the orbit and does not fall into the nucleus.
9. How did Rutherford conclude that an atom contains equal number of protons and electrons?
Ans. The presence of negatively charged electrons and positively charged protons in an atom had been proved experimentally. Also, a proton and an electron have equal but opposite charges. As an atom is electrically neutral, Rutherford concluded that an atom should contain equal number of protons and electrons.
10. which discovery became possible from the findings of a -particle scattering experiment?
Ans. From α -particle scattering experiment it was proved that, all the positive charge and mass of an atom is concentrated in a very small region within the atom. This small region is known as nucleus. Thus, discovery of nucleus became possible due to α -particle scattering experiment.
11. What would have happened if Rutherford had used a foil of a lighter metal than gold foil in his a-scattering experiment?
Ans. The relative mass of an α -particle is 4 units. In α-scattering experiment, instead of gold foil, if a foil of any other lighter metal was used, then the α-particle might have passed through the foil without any deflection. As a result, scattering of α -particles would not have taken place.
12. Which observation led to the discovery of neutrons in an atom?
Ans. Except ordinary hydrogen, the masses of all other atoms were found to be heavier than the total mass of electrons and protons in the atoms. This observation forced scientists to think about the presence of any other neutral particle having fixed mass in the atom. This ultimately led to the discovery of neutrons in an atom.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. Which metal plate was used in Rutherford’s α-scattering experiment?
A. aluminium
B. gold
C. silver
D. zinc
Ans. B
2. The existence of which of the following particles of an atom was proved by Rutherford’s α -scattering experiment?
A. electron
B. proton
C. nucleus
D. neutron
Ans. C
3. An alpha (α) particle is
A. positively charged hydrogen ion
B. unipositive helium ion
C. dipositive helium ion
D. a chargeless particle
Ans. C
4. Which of the following elements is an α-particle emitter?
A. radium
B. iron
C. lead
D. bismuth
Ans. A
5. In Rutherford’s experiment, most of the α-particles passed straight through the thin metallic foil without suffering any deflection. The reason is that
A. α-particles are very small in size with respect to electrons
B. α-particles are positively charged
C. most of the part inside an atom is empty
D. α -particles are slow moving particles
Ans. C
6. The nucleus of an atom is
A. positively charged
B. negatively charged
C. neutral
D. partly positive and partly negative
Ans. A
7. According to Rutherford’s atomic model, the entire mass of an atom
A. is uniformly distributed throughout the atom
B. is concentrated within the nucleus
C. is distributed outside the nucleus
D. remains partly within the nucleus and partly outside the nucleus
Ans. B
8. According to Rutherford’s atomic model, protons are
A. present within the nucleus
B. present outside the nucleus
C. revolving around the nucleus
D. none of these
Ans. A
9. Electrons present in an atom
A. remain static within the nucleus
B. remain static outside the nucleus
C. revolve around the nucleus
D. revolve within the nucleus
Ans. C
10. The inference which cannot be drawn from Rutherford’s α-scattering experiment is that
A. the nucleus is small and heavy
B. the nucleus is always positively charged
C. the size of an atom is almost 105 times that of the nucleus
D. the nucleus was hit by a large number of α -particles
Ans. D
11. An accelerated charged particle
A. absorbs energy
B. emits energy
C. neither absorbs nor emits energy
D. initially absorbs energy and then emits it
Ans. B
12. Which of the following atoms does not have any neutron in it?
A. protium
B. deuterium
C. tritium
D. helium
Ans. A
Answer in brief
1. Which particles did Rutherford use for his scattering experiment?
Ans. Alpha (α) particles.
2. Name the different rays that are emitted from radioactive elements.
Ans. Alpha (α) rays, beta (β) rays and gamma (γ) rays.
3. What is the ratio of penetrating powers of α, β and γ-rays?
Ans. The ratio of penetrating powers of α , β and γ-rays is approximately 1:100:1000.
4. What is the nature of γ-ray, obtained as a result of radioactive radiation?
Ans. γ-ray is an electromagnetic radiation.
5. Mention nature of α -particle.
Ans. α -particle is just like helium nucleus bearing 2 units of positive charge and 4 units of mass.
6. What is the approximate measure of the diameter of nucleus of an atom?
Ans. 10-12 to 10-13 cm.
7. How is the mass arranged in an atom according to Rutherford’s atomic model?
Ans. According to Rutherford’s atomic model almost all of the mass are centralised at the nucleus.
8. What is the name of the heaviest particle present in an atom?
Ans. Neutron.
9. What is the diameter of an atom?
Ans. The diameter of an atom is approximately 10-8 cm.
10. Give an example of electromagnetic radiation.
Ans. Visible light.
11. Who coined the term neutron?
Ans. Scientist Rutherford.
12. What is the mass of a neutron particle? .
Ans. Mass of a neutron particle is 1.675 × 10-27 kg
13. Who discovered neutrons?
Ans. Sir James Chadwick (In 1932).
14. What is the nature of atomic spectra?
Ans. Atomic spectra is discontinuous in nature and is basically a line spectra.
15. Name the charge-less or neutral subatomic particle.
Ans. Neutral subatomic particle is neutron.
16. Do the electrons remain static within an atom?
Ans. According to Rutherford’s atomic model, electrons revolve around the nucleus in circular paths and thus, do not remain static within an atom.
17. If a charged particle moves with accelerated motion, then what will be the change in its energy?
Ans. If a charged particle moves with accelerated motion, it will continuously emit radiation. Hence, the energy of the particle will gradually decrease.
Fill in the blanks
1. Most of the part inside an atom is …………..
Ans. empty
2. The positively charged heavy part of an atom is known as the …………..
Ans. nucleus
3. Scientist who carried out the a-particle scattering experiment was …………..
Ans. Rutherford
4. An electron is attracted towards the nucleus by …………… force of attraction.
Ans. electrostatic
5. The number of ………….. in the nucleus is equal to the number of ………….. revolving around the nucleus in different orbits.
Ans. protons, electrons
6. Rutherford’s atomic model fails to explain the ………….. of an atom.
Ans. stability
7. The mass of a neutron is nearly equal to the mass of a …………..
Ans. proton
8. The charge of a neutron is …………..
Ans. zero
State whether true or false
1. Size of the nucleus is extremely small compared to the size of an atom.
Ans. True
2 Radioactive elements emit alpha, beta and gamma rays.
Ans. True
TOPIC – C
Bohr-Rutherford’s Atomic Model
SHORT AND LONG ANSWER TYPE QUESTIONS
1. State the major postulates of BohrRutherford atomic model.
Ans. The major postulates of Bohr-Rutherford atomic model are as follows- (1) Electrons do not revolve around the nucleus in any circular orbit. Instead, they revolve only in some selected orbits of definite radii. (2) While revolving along such an orbit, an electron neither emits nor absorbs any form of energy, i.e., the energy of the electron remains constant. So, these orbits are known as stationary orbits. (3) An electron absorbs or emits energy only when it jumps from one orbit to another. When an electron jumps from a higher energy orbit to a lower energy orbit, it emits energy. On the other hand, an electron jumps from a lower energy orbit to a higher energy orbit by absorbing energy.
2. What are stationary orbits? Why are they called so?
Ans. According to Bohr’s atomic model, the circular paths along which electrons revolve around the nucleus are called stationary orbits..
When an electron moves along such an orbit, it neither emits nor absorbs energy and hence the energy of the electron remains fixed. So these orbits are called stationary orbits.
3. State the significance of BohrRutherford atomic model.
Ans. According to Bohr-Rutherford atomic model, electrons moving in stationary orbits around the nucleus neither absorb nor emit energy continuously. Hence, the energy of electrons in those orbits remains constant. Thus, the theory successfully explains the stability of an atom which Rutherford’s theory failed to explain.
4. What is meant by ground state and excited state of an atom?
Ans. Ground state: At normal condition, the revolving electrons in an atom occupy the lowest energy orbits. This state of an atom is called its ground state.
Excited state: When the electrons absorb energy from the surroundings in the form of heat or light, the electrons jump from lower energy orbits to higher energy orbits. This state of an atom is called its excited state.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. In an atom, there are
A. equal number of neutrons and protons
B. equal number of protons and electrons
C. equal number of neutrons and electrons
D. equal number of protons and positrons
Ans. B
2. Mass number of an atom is usually expressed by the alphabet
A. X
B. M
C. Z
D. A
Ans. D
3. Atomic number of an atom is usually expressed by the alphabet
Ans. C
4. The circular paths along which electrons revolve around the nucleus are known as
A. ground state
B. stationary orbits
C. excited state
D. stationary state
Ans. B
5. The lowest energy state of an atom is known as its
A. ground state
B. equilibrium state
C. excited state
D. stationary state
Ans. A
6. By absorbing energy, an electron
A. jumps to an outer orbit
B. jumps to an inner orbit
C. does not change its orbit
D. revolves faster
Ans. A
7. Mass number of an atom indicates the total number of
A. protons and electrons
B. neutrons
C. protons and neutrons
D. protons, neutrons and electrons
Ans. C
8. Number of neutrons present in deuterium
A. 0
B. 1
C. 2
D. 3
Ans. B
9. According to Bohr’s atomic model number of stationary orbits is
A. 9
B. 5
C. 7
D. 11
Ans. C
10. If the total number of electrons present in a trivalent cation is 10, its atomic number is
A. 10
B. 7
C. 13
D. 14
Ans. C
Answer in brief
1. Bohr’s theory is not applicable for which of the given species- H, H+, He+, Li2+?
Ans. Bohr’s atomic model is applicable only for single electron system. As H+ does not have any electron, Bohr’s theory is not applicable for this ion.
2. What is an orbit?
Ans. According to Bohr-Rutherford atomic model, an orbit is the circular path around the nucleus along which the electrons revolve around the nucleus in an atom.
3. What is atomic number?
Ans. Atomic number of an atom is defined as the number of protons present in the nucleus of an atom.
4. Wh mass number?
Ans. Mass number of an atom is the sum of the number of protons and neutrons present in the nucleus of an atom.
5. What are the names of the Bohr orbits in increasing order of their distance from the nucleus?
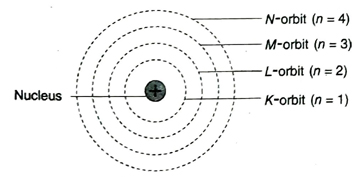
Ans. According to increasing order of their distance from the nucleus, the seven Bohr orbits are named as K, L, M, N, O, P and Q orbits.
6. How does the energy of the orbit change on moving away from the nucleus?
Ans. On moving away from the nucleus, the energy of the orbits gradually increases.
7. How does an atom absorb or emit energy?
Ans. The absorption or emission of energy by an atom always takes place discontinuously. (Absorption or emission of energy always takes place in an integral multiple of the smallest unit of energy, called quantum)
8. What happens when an electron absorbs excess energy?
Ans. When an electron absorbs excess energy, it jumps from a lower energy orbit to a higher energy orbit. (If sufficient energy is supplied, then the electron may be removed from the atom.)
9. Which theory forms the basis of BohrRutherford’s atomic model?
Ans. Quantum theory.
10. What is the minimum number of orbits that an atom can have?
Ans. An atom may have minimum 1 orbit.
11. Write down the relation between atomic number and mass number.
Ans. Mass number = Atomic Number + Number of neutrons.
12. There are 11 protons in an atom. Find the number of electrons in the uni-positive ion of that atom.
Ans. The atom contains 11 protons. So the umber of electrons in the uni-positive ion of that atom will be 10.
13. If an atom contains 8 protons, then find the number of electrons in the di-negative ion of that atom.
Ans. There are 8 electrons in the said atom. Hence, the di-negative ion of that atom will contain 10 electrons.
Fill in the blanks
1. Maximum number of electrons that can be present in a principal energy level is ………..
Ans. 2n2
2. Bohr-Rutherford atomic model is ……….. in nature.
Ans. two-dimensional
3. Atomic number is always a ………… number.
Ans. whole
4. The circular path along which an electron revolves around the nucleus is known as ………….
Ans. orbit
5. The orbits whose energy remain fixed are called ……….. orbits.
Ans. stationary
6. When an electron jumps to a higher energy level by absorbing energy, it is said that the atom is in its ………. state.
Ans. excited
7. Atomic number indicates the number of ………… in an atom.
Ans. protons
State whether true or false
1. Atomic number of the element M is 13. Thus, number of electrons in M³- ion will be 10.
Ans. False
2. Bohr’s atomic model is applicable for multielectron systems.
Ans. False
3. Bohr’s atomic model is unable to give an idea about the actual three-dimensional electronic model of an atom.
Ans. True
4. Mass number = Number of protons + Number of electrons.
Ans. False
5. An electron neither emits nor absorbs energy while moving along a stationary orbit.
Ans. True
TOPIC – D
Isotope, Isobar, Isotone, Nuclear Force and Idea of Electronic Configuration
SHORT AND LONG ANSWER TYPE QUESTIONS
1. Why do isotopes of the same element possess identical chemical properties?
Ans. Chemical properties of an element depend on its atomic number and the electronic configuration of the element. Isotopes of the same element may have different number of neutrons, but they possess the same number of protons and also have identical electronic configuration. Thus, isotopes of the same element have identical chemical properties.
2. What are the different characteristic features of isobars?
Ans. Different characteristic features of isobars are- (1) Isobars are atoms of different elements. (2) They have different atomic numbers. (3) They have the same mass number. (4) They have different physical and chemical properties. (5) Isobars have different number of protons, neutrons as well as electrons.
3. What is nuclear force? How does this force originate?
Ans. The powerful attractive force that acts among the nucleons (protons and neutrons) at a very short range (at a distance of about 1.5 fermi) inside the nucleus is known as nuclear force. It is responsible for holding the nucleons together within the nucleus.
In 1935, scientist Hideki Yukawa proposed ‘meson theory’ to explain the origin of nuclear force. According to this theory, protons and neutrons in the nucleus constantly exchange a small particle called л-meson. Due to the exchange of these meson particles, protons get converted to neutrons and vice-versa. As a result, the repulsion between protons become insignificant and a strong attractive force is created between the nucleons. This is how the nuclear force originates.
4. Mention the different characteristics of different characteristics of nuclear force.
Ans. Different characteristics of nuclear force are- (1) Nuclear force is a strong attractive force that holds the nucleons within a close space inside the nucleus. (2) The nature of nuclear force is quite different from that of gravitational force or electrostatic force of attraction. (3) Nuclear force is almost 1040 times stronger than gravitational force while it is 100 times stronger than coulombic force. (4) Nuclear force is charge independent. The nature of forces acting between two protons or two neutrons or between a proton and a neutron are identical. (5) This is a very short range force. The force acts within a range of approximately 1 × 10-15 m within the nucleus. As a result, the force does not exist outside the nucleus. (6) With the help of this force, each nucleon gets attracted only by its nearest neighbouring nucleons.
5. What is Bohr-Bury scheme?
Ans. The Bohr-Bury scheme explains the arrangement of electrons in different orbits around the nucleus. This arrangement is known as electronic configuration. The Bohr-Bury rules regarding electronic configuration of an atom in different orbits are as follows- (1) The n-th orbit of an atom accommodates a maximum of 2n² electrons where ‘n’ represents the principal quantum number. (2) The maximum possible number of electrons in the outermost orbit of an atom is 8 irrespective of the principal quantum level. (3) An electron does not occupy a higher energy orbit while a lower energy orbit is left vacant.
6. Is it possible to write the electronic configuration of an atom from the atomic number of the element? Justify your answer.
Ans. Yes, it is possible to write the electronic configuration of an atom if the atomic number of the element is known.
An atom is electrically neutral and the total positive charge is equal to the total negative charge. Hence, the number of protons in an atom is equal to the number of electrons. Thus, if atomic number of the element is known, the number of protons as well as number of electrons will be known and hence we can write the electronic configuration of the atom..
7. The atomic number of sodium is 11. Write the electronic configuration of unipositive ion of sodium (Na+).
Ans. The atomic number of sodium is 11. Hence, there are 11 electrons in a sodium atom and its electronic configuration is 2,8,1. Na atom forms Na+ ion by releasing an electron from its valence shell. Hence, the electronic configuration of Na+ ion is 2,8.
8. The atomic number of chlorine is 17. Write the electronic configuration of uninegative ion of chlorine (Cl–).
Ans. The atomic number of chlorine is 17. Hence, there are 17 electrons in a Cl- atom and its electronic configuration is 2,8,7. Cl– atom forms CI-ion by accepting an electron and number of electrons in its valence shell increases by 1. Thus, the electronic configuration of Cl– ion is 2,8,8.
9. Maximum number of electrons that can be accommodated in M-orbit is 18 but the electronic configuration of potassium (atomic number = 19) cannot be written as 2,8,9. Why?
Ans. The outermost orbit of an atom cannot accommodate more than 8 electrons. So, M orbit being the outermost orbit can accommodate a maximum of 8 electrons. Thus, the 19th electron of potassium atom enters into N orbit. Therefore, electronic configuration of potassium atom is 2,8,8,1 and not 2,8,9.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. The number of isotopes of oxygen is
A. 3
B. 4
C. 1
D. 2
Ans. A
2. An element which has no isotope is
A. carbon
B. chlorine
C. fluorine
D. oxygen
Ans. C
3. Isotopes of an element possess
A. identical physical properties
B. different chemical properties
C. different number of neutrons
D. different atomic numbers
Ans. C
4. Isotopes contain equal number of
A. neutrons
B. protons
C. positrons
D. mesons
Ans. B
5. Isobars have identical
A. atomic number
B. number of protons
C. number of electrons
D. mass number
Ans. D
6. Isotones have identical
A. number of electrons
B. number of protons
C. number of neutrons
D. number of positrons
Ans. C
7. For isotopes, a slight difference is observed in case of their
A. actual mass
B. atomic number
C. number of electrons
D. number of protons
Ans. A
8. Protons and neutrons of an atom are collectively known as
A. positron
B. isotope
C. nucleon
D. isobar
Ans. C
9. The range within which nuclear force acts is
A. 1.5 fermi
B. 2.5 fermi
C. 3.5 fermi
D. 4.5 fermi
Ans. A
10. Maximum number of electrons that can be accommodated in the valence shell of an atom is
A. 18
B. 10
C. 2
D. 8
Ans. D
11. Which of the following noble gases does not contain 8 electrons in its valence shell?
A. Ne
B. Ar
C. Xe
D. He
Ans. D
12. The atoms and ions with equal number of electrons are known as
A. isotopes
B. isobars
C. isotones
D. isoelectronic
Ans. D
13. Number of electrons in S2- ion
A. 15
B. 16
C. 17
D. 18
Ans. D
14. The element which does not contain 8 electrons in the outermost orbit
A. Ar
B. Ne
C. Pd
D. none of these
Ans. C
Answer in brief
1. Who discovered radioactivity?
Ans. French scientist Henri Becquerel.
2. What similarities are observed in the isotopes of an element?
Ans. All the isotopes of an element have the same atomic number and identical chemical properties.
3. What similarities are observed in isobars?
Ans. Isobars have the same mass number.
4. What similarities are observed in isotones?
Ans. Isotones contain same number of neutrons in their nucleus.
5. Exchange of which particle between protons and neutrons is responsible for the generation of nuclear force?
Ans. Exchange of meson particles.
6. Who explained the formation of nuclear force?
Ans. Scientist Yukawa .
7. What is meant by valence shell of an atom?
Ans. The outermost orbit of an atom is known as the valence shell of that atom.
8. Name the inert gas which has two electrons in its valence shell.
Ans. Helium (He) is the inert gas which has two electrons in its valence shell.
9. State the maximum number of electrons that can be accommodated by the valence shell of an atom.
Ans. The maximum number of electrons that can be accommodated by the valence shell of an atom is 8.
10. What is meant by electronic configuration of an atom?
Ans. In a multi-electron atom, the definite arrangement of electrons in different orbits around the nucleus is known as its electronic configuration.
11. What is the maximum possible number of electrons that can be accommodated by the M-orbit of an atom?
Ans. The M-orbit of an atom can accommodate a maximum of 18 electrons.
12. Who was the first to observe the difference in number of neutrons in different atoms of the same ment (or the existence of isotopes)?
Ans. Scientist Soddy.
13. Give an example of an element which has no naturally occurring isotope.
Ans. Elements such as fluorine (F) and sodium (Na) have no naturally occurring isotope.
14. Which element has the maximum number of stable isotopes?
Ans. Tin (Sn) has the maximum number (10) of stable isotopes.
15. How does the energy of an orbit vary with increasing distance from nucleus?
Ans. Energy of an orbit increases with the increase in distance from the nucleus.
16. An atom has three orbits. The third orbit contains 5 electrons. What is its atomic number?
Ans. Hence, the electronic configuration of the atom is K(2), L(8), M(5).
∴ Its atomic number is (2+8+5) = 15.
17. How many electrons are there in the Mshell of potassium (Z = 19) ?
Ans. Electronic configuration of potassium is K(2), L(8), M(8), N(1). So number of electron in M-shell is 8.
18. Which subatomic particle is responsible for formation of isotope?
Ans. Neutron.
19. What is the possible maximum number of electrons in the n-th orbit of an atom?
Ans. Possible maximum number of electrons in the n-th orbit of an atom is 2n².
20. What is formed when electrons are removed from an atom?
Ans. Positively charged ion or cation is formed when electrons are removed from the outermost shell of an atom.
Fill in the blanks
1. Isotopes are identical with respect to their ………… properties.
Ans. chemical
2. Isobars have the same ……….. number.
Ans. mass
3. Isotones contain equal number of …………
Ans. neutrons
4. Nuclear force operates within a range of ………… m.
Ans. 1.5 × 10-15
5. Nuclear force originates due to the exchange of ………… particles between protons and neutrons.
Ans. π-meson
6. Most of the naturally occurring elements exist as a mixture of two or more of their ………….
Ans. isotopes
7. Isotopes of an element have the …………. electronic configuration.
Ans. same
8. Electronic configurations of isobars are ………….
Ans. different
9. Except helium, all other noble gases contain ………….. electrons in their valence shell.
Ans. 8
10. The filling of electrons in their respective orbits obeys the ………… rule.
Ans. Bohr-Bury
State whether true or false
1. The n-th orbit around the nucleus can accommodate a maximum of 2n2 electrons.
Ans. True
2. Isotopes are atoms of the same element with the same atomic number but different mass numbers.
Ans. True
3. The M-orbit of an electron can accommodate a maximum of 8 electrons.
Ans. False
4. Atomic numbers of elements P and Q are 18 and 20 respectively whereas their mass number is 40. P and Q are isotones.
Ans. False
5. Nuclear force is a long range force.
Ans. False
6. Outermost orbit of neon does not contain 8 electrons.
Ans. False