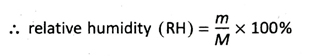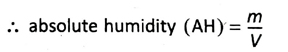WBBSE 9th Class Science Solutions Physical Science & Environment Chapter – 6 Heat
When several bodies are at thermal equilibrium the physical quantity ‘temperature’ is same for all of the bodies.
A. 5:1
B. 1:5
C. 2:3
D. 4:3
A. changed form of momentum
B. changed form of kinetic energy
C. changed form of potential energy
D. changed form of velocity
A. temperature of a body
B. latent heat
C. radiated heat
D. all of the above
A. directly proportional only to the mass
B. directly proportional only to the rise of temperature
C. directly proportional to the product of mass and increased temperature
D. directly proportional to the quotient of mass and increased temperature
A. heat has to enter the body or the system from outside
B. heat has to go out of the body or the system
C. a chemical reaction has to take place in the body or the system
D. one body should not be soluble in another body
A. water
B. iron
C. air
D. mercury
A. difference in water equivalent
B. difference in heat capacity
C. difference in heat conduction
D. difference in specific heat
A. mass of the body is variable
B. mass of the body is fixed
C. water equivalent of the body is variable
D. latent heat of change of state of the body is constant
A. specific heat of water is greater than that of iron
B. specific heat of iron is greater than that of water
C. iron gets heated slowly compared to water
D. water releases heat faster than iron
A. the temperature of the body
B. the change of temperature of the body
C. the total time of heating
D. all of the above
A. 40°C
B. 50°C
C. 60°C
D. 80°C
A. glass
B. copper
C. gold
D. wood
A. water
B. alcohol
C. kerosene
D. milk
A. heat
B. temperature
C. mechanical energy
D. internal energy
A. sum of the temperatures of the bodies
B. total heat of the bodies
C. total internal energies of the bodies
D. internal energy of each body
A. specific heat capacity and heat
B. heat capacity and water equivalent
C. specific heat capacity and heat capacity
D. heat and work
A. mass
B. density
C. specific heat capacity
D. increase in temperature
SHORT AND LONG ANSWER TYPE QUESTIONS
1. Write and explain Joule’s law regarding equivalence of work and heat.
Ans. If work can be fully converted to heat, then work done and heat produced are directly proportional to each other. This is Joule’s law. Suppose, W amount of work is done to produce H amount of heat. According to Joule’s law,
W ∝ H or, W = JH
where J is a constant and is called mechanical equivalent of heat.
2. Define mechanical equivalent of heat. What is its value in erg/cal?
Ans. Mechanical equivalent of heat is defined as the amount of work that has to be done to produce one unit of heat.
Value of J in CGS system is 4.2 × 107 erg/cal, this may be expressed as 4.2 J/cal.
3. Heat is produced when work is done-give an example.
Ans. When a wooden door or any material is polished with a piece of cloth by applying some pressure, we find that both our hand and the cloth warm up considerably. Polishing means reducing the roughness of the surface to make it more smooth. Work done against friction during polishing gets converted into heat.
4. Water in a waterfall falls from a height to the ground. Why is the temperature of water at the ground level slightly more than that at the top?
Ans. When water from the top of the waterfall falls towards the ground, its potential energy decreases. This decrease of potential energy is converted into kinetic energy. After water strikes the ground, a portion of this kinetic energy is converted to heat energy. Now, a portion of this generated heat remains confined to water to increase its temperature. For this reason, temperature at the ground level is slightly higher than that at the top.
5. If a chunk of ice is dropped from a distant height, then why does a portion of it melt after striking the ground?
Ans. At normal temperature, if a chunk of ice at 0°C is dropped from a distant height to the ground, its potential energy decreases. This decrease of potential energy is converted into kinetic energy. After it strikes the ground, a portion of this kinetic energy is converted to heat energy. Apart from this, heat is also generated due to its friction with air. Now a part of this produced heat remains confined to ice and melts a portion of the ice.
6. What do you mean by the state or phase www. of a material?
Ans. A material may exist in different forms. These forms are physically distinct from each other but have the same chemical composition. By absorption or release of heat or by any other mechanical means, forms of a material can be changed. Each form of the material is known as a distinct state or phase.
7. What is the change of state of a t is the material? How many types of change are envisaged on the basis of absorption or release of heat? What are those changes?
Ans. Change of state of a material is defined as the phenomenon of transformation of a material from one state to another due to either absorption or release of definite amount of heat to or from a material.
Two types of change are envisaged.
The changes are: (1) higher change of state and (2) lower change of state.
8. What do you mean by higher change of It do you mean by higher change of state?
Ans. A change of state of a material due to application of heat is called higher change of state.
A solid material can be converted to a liquid material and a liquid material can be converted to a gaseous material by absorption of heat. Transformation from solid to liquid state is called melting and transformation from liquid, to gaseous state is called vaporisation. Thus, melting and vaporisation are higher change of state.
9. What do you mean by lower change of hat do you mean by lower chan state?
Ans. A change of state of a material due to release of heat is called lower change of state.
A liquid material can be converted to a solid material and a gaseous material can be converted to a liquid material by release of heat. Transformation from liquid to solid state is called solidification and transformation from gaseous to liquid state is called condensation. Thus, solidification and condensation are lower change of state.
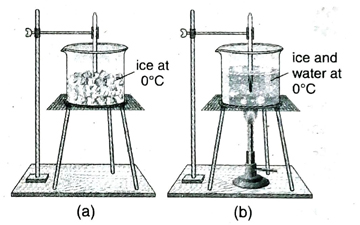
10. With th the help of an experiment, demonstrate that temperature remains constant during the time of change of state.
Ans. Some amount of ice is taken in a vessel. Now a thermometer is clamped by its side and its bulb is immersed in ice as shown in Fig. (a). Reading of the thermometer is now 0°C. Now the vessel is heated slowly. Ice starts melting due to absorption of heat as shown in Fig. (b). It is observed that till the total amount of ice melts away, the reading of the thermometer remains constant at 0°C. After the total amount of ice melts in water, it is observed that the reading of the thermometer starts increasing. It is inferred from this experiment that the temperature remains constant during the time of change of state.
11. What do you mean by latent heat?
Ans. During the period of change of state of any material, whether there is any absorption or release of heat, there is not any change of temperature. The amount of heat released or absorbed per unit mass of a substance during change from one state to another by keeping the pressure constant is called the latent heat of the substance for the constant change of state.
12. What do you understand by the statement: Latent heat of melting of ice is 80 cal/g.
Ans. Latent heat of melting of ice is 80 cal/g means that under normal pressure, 80 cal of heat is required to convert 1 g of ice at 0°C to 1 g of water at 0°C.
13. what do you understand by the statement: Latent heat of vaporisation of water is 537 cal/g.
Ans. Latent heat of vaporisation of water is 537 cal/g means that under normal pressure, 537 cal of heat is required to convert 1 g of water at 100°C to 1 g of vapour at 100°C.
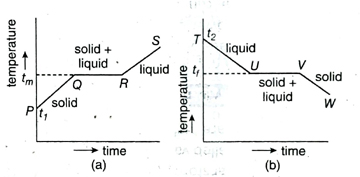
14. Give a graphical explanation of latent heat.
Ans. A solid material is taken. Suppose the initial temperature of the material is t₁. In Fig. (a), point P indicates initial temperature of the material. If heat is supplied to the material at a definite rate, its temperature increases. This is represented by the line PQ in the graph. Then the material starts melting. Point Q indicates beginning of melting. Temperature of point Q is the melting temperature tm. Now, if heat is given continuously, the material melts and till the melting is complete, temperature remains constant. Line QR in the graph represents melting of the material. Melting is complete at point R where the entire solid material has been converted to liquid. Now if further heat is given, temperature of the liquid increases. Line RS in the graph represents this increase.
In the same way, we may take a liquid material with initial temperature of t2 and start releasing heat at a definite rate. Change of temperature with time is depicted in Fig. (b) where point T denotes the initial temperature of the liquid. Reduction of temperature of the liquid is expressed by line TU. At point U, solidification of liquid starts and it ends at point V.
Temperature remains unchanged from U to V. At point V, the entire liquid material has been converted to solid material. After this, if heat release goes on, temperature of the solid is decreased which is represented by line VW.
QR portion and UV portion in the Fig. (a) and Fig. (b) represent the latent heat of absorption and latent heat of release, respectively. These portions of the lines are straight lines parallel to the time axis because in these two cases, temperature remains unchanged.
15. Why does ice at 0°C feel colder than water at 0°C?
Ans. Under normal pressure, 1 g of ice at 0°C is obtained after release of 80 cal of heat from 1 g of water at 0°C. This means that 1 g of ice at 0°C contains 80 cal less heat than 1 g of water at 0°C. Therefore, ice at 0°C feels colder.
16. Why does steam at 100°C feel warmer at 100°C feel w than water at 100°C?
Ans. Under normal pressure, 1 g of steam at 100°C is obtained after applying 537 cal of heat to 1g of water at 100°C. This means that 1g of steam at 100°C contains 537 cal more heat than 1g of water at 100°C. For this reason, steam at 100°C feels warmer than water at 100°C.
17. Why is latent heat of vaporisation higher than latent heat of melting?
Ans. There are strong intermolecular forces working amongst the molecules in a solid matter. Compared to these, intermolecular forces in liquid are less and in gases, these forces are significant. When converted from liquid to gaseous state, intermolecular distances between the molecules increase more than when it is converted from solid to liquid state. As a result, during transformation from liquid to gaseous state, more work is to be done against this intermolecular attractive forces, requiring more heat. For this reason, latent heat of vaporisation is higher than latent heat of melting.
18. Why is water kept in an ice-tray at 0°C not converted into ice?
Ans. Water is kept in an ice-tray. If temperature of water is more than 0°C, then that water releases heat to become water at 0°C and some amount of ice melts in the process. At this state, as temperature of ice and water are at 0°C, there is no exchange of heat. So, when water is kept in an ice-tray, it does not get converted to ice.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. Amount of heat required to convert 100 g of water at a temperature of 100°C to 100 g of steam at a temperature of 100°C is (latent heat of vaporisation = 540 cal/g)
A. 5400 cal
B. 540000 cal
C. 27000 cal
D. 54000 cal
Ans. D
2. If mechanical equivalent of heat, J = 4.2 J/cal, how much work has to be performed to produce 5 cal of heat?
A. 20 J
B. 42 J
C. 21 J
D. 8.4 J
Ans. C
3. During the time of higher change of state
A. heat is absorbed
B. heat is released
C. heat is neither applied nor released
D. temperature increases
Ans. A
4. During the time of lower change of state
A. heat is absorbed
B. heat is released
C. heat is neither absorbed nor released
D. temperature decreases
Ans. B
5. At any point of time during absorption of heat
A. only change of state takes place
B. only change of temperature takes place
C. change of state or temperature takes place
D. both change of state and temperature take place
Ans. C
6. Due to release of heat, electrical conductivity of a body
A. decreases
B. increases
C. remains constant
D. sometimes increases, sometimes decreases
Ans. C
7. According to scientists Rumford and Davy, which energy is converted to heat energy?
A. chemical energy
B. electrical energy
C. mechanical energy
D. potential energy
Ans. B
8. When mechanical energy is converted to heat energy, then
A. kinetic energy of the body increases
B. kinetic energy of atoms and molecules of the body increases
C. water equivalent of the body increases
D. specific heat of the body increases
Ans. B
9. Amount of heat required to melt ice of 1 g into water at 0°C is
A. 100 cal
B. 500 cal
C. 4 cal
D. 80 cal
Ans. D
10. Amount of heat required to convert 1 g of water into vapour at 100°C is
A. 537 cal
B. 100 cal
C. 80 cal
D. 4 cal
Ans. A
11. On the upper surface of an uncovered liquid
A. condensation = vaporisation
B. condensation > vaporisation
C. condensation < vaporisation
D. data incomplete
Ans. C
12. If there is no change of state, then which of the following quantity is unnecessary for calculation of absorption or release of heat?
A. mass
B. latent heat
C. specific heat
D. change of temperature
Ans. B
13. If heat is applied to dry ice (solid carbon dioxide), then it is directly converted to gaseous carbon dioxide. This phenomenon is called
A. vaporisation
B. boiling
C. sublimation
D. melting
Ans. C
14. If heat is applied to water at 0°C, then
A. density increases at first and then decreases
B. volume increases at first and then decreases
C. volume increases continuously
D. mass increases at first and then decreases
Ans. A
15. A piece of ice is floating in a glass of water at 3°C. If this ice melts completely, level of water in the glass
A. decreases
B. increases
C. remains same
D. is unpredictable
Ans. B
16. A piece of ice is floating in a glass of water at 5°C. Melting of the piece of ice decreases the temperature of water to 4°C. At this condition, level of water
A. decreases
B. increases
C. remains same
D. data incomplete
Ans. A
17. On the upper surface of a liquid kept in a closed vessel
A. rate of condensation > rate of vaporisation
B. rate of condensation < rate of vaporisation
C. rate of condensation = rate of vaporisation
D. rate of condensation ≥ rate of vaporisation
Ans. C
18. Vaporisation of water takes place
A. at 0°C
B. at 15°C
C. only at 100°C
D. at any temperature from 0°C to 100°C
Ans. D
19. At what temperature latent heat is required for the conversion of water to vapour?
A. at 0°C
B. at 14.5°C
C. only at 100°C
D. at any temperature between 0°C and 100°C
Ans. D
20. A phenomenon or state which does not depend on the amount of water vapour present in air is
A. falling of dew
B. formation of fog
C. freezing of water to form ice
D. relative humidity
Ans. C
21. Latent heat of vaporisation of water
A. depends on the amount of heat absorbed
B. depends on the mass of water
C. depends on the volume of water
D. does not depend on anything
Ans. D
22. Which of the following is a higher change of state?
A. solidification
B. vaporisation
C. condensation
D. none of these
Ans. B
23. Which of the following is a lower change of state?
A. melting
B. vaporisation
C. condensation
D. none of these
Ans. C
Answer in brief
1. Some amount of heat is required for change of state of a material. What is that heat called?
Ans. The heat that is required for change of state of a material is called latent heat.
2. What is the unit of latent heat in CGS system?
Ans. Unit of latent heat in CGS system is cal/g.
3. What type of change of state is represented by melting and vaporisation?
Ans. Melting and vaporisation represent higher change of state.
4. What type of change of state is represented by solidification and condensation?
Ans. Solidification and condensation represent lower change of state.
5. What is the value of mechanical equivalent of heat in CGS system?
Ans. Value of mechanical equivalent of heat in CGS system is 4.2 × 107 erg/cal.
6. If there is no change of temperature of a body in spite of heating, can there be a change of state of the body?
Ans. Yes, there may not be a change of temperature of a body but due to application of heat, there may be a change of state of the body.
7. What is sublimation?
Ans. Sublimation is the process by which a solid material is converted directly to its gaseous state due to absorption of heat.
8. What is the water equivalent of a body if its heat capacity is 50 cal/°C?
Ans. Water equivalent of the body is 50g.
9. If work done is fully converted to heat, what is the relationship between work done and heat produced?
Ans. Work done and heat produced are directly proportional to each other.
10. Name four fundamental states of matter. ,
Ans. Four fundamental states of matter are solidliquid, gaseous and plasma state.
11. To produce 1 kcal heat how much work is needed?
Ans. The amount of work is needed to produce 1 kcal of heat is = 1000 × 4.2 J = 4200 J.
12. What is the value of latent heat of fusion of ice in CGS system?
Ans. The value of latent heat of fusion of ice in CGS system is 80 cal/g.
13. What is the specific heat of water during boiling at 100°C?
Ans. During boiling at 100°C specific heat of water is infinite.
14. What is latent heat of condensation?
Ans. The amount of heat extracted from unit mass of a vapour to change it into its liquid state at a constant temperature, is the latent heat of condensation or the vapour.
15. Why steam at 100°C is a better warming agent than water at 100°C?
Ans. 1 g of steam at 100°C can transfer 537 cal more heat than 1 g of water at 100°C. That is why steam at 100°C is a better warming agent than water at 100°C.
16. Under what condition can heat be supplied to a body without causing any change in temperature?
Ans. During change from one state to other the substance absorbs heat without any change in the temperature.
Fill in the blanks
1. ………. is that amount of work which needs to be done to produce one unit of heat.
Ans. mechanical equivalent of heat
2. Dimension of L in the dimensional formula of latent heat is …………
Ans. 2
3 On the basis of absorption or release of heat, change of state of any material is of …………. types.
Ans. two
4. Water falls from top to bottom in a waterfall. Compared to the water at top, temperature is slightly ………… at the bottom.
Ans. higher
5. Water kept in the ice tray at 0°C …………. temperature convert into ice.
Ans. does not
6. Matter requires latent heat for any change of ………….
Ans. state
7. Latent heat of melting of ice is …………
Ans. 80 cal/g
8. An amount of 8.4 J work has to be done to produce …………. of heat.
Ans. 2 cal
9. ………….. of liquid takes place at any temperature.
Ans. vaporisation
10. Rate of vaporisation ………….. with increase of temperature of liquid.
Ans. increases
State whether true or false
1. Latent heat of melting is greater than latent heat of vaporisation.
Ans. True
2. Heat is the transformed state of the kinetic energy of a particle.
Ans. True
3. In steam engine heat is converted into mechanical work.
Ans. True
4. Steam at 100°C causes severe burn than water at 100°C.
Ans. True
5. The gradient of temperature vs time graph of a substance have – ve value when heat is supplied to it.
Ans. False
6. The gradient of temperature vs time graph of a substance have – ve value when heat is extracted from it.
Ans. True
7. Sublimation is direct conversion from solid to gaseous state.
Ans. True
TOPIC – C
Saturated, Unsaturated Vapour and Anomalous Expansion of Water
SHORT AND LONG ANSWER TYPE QUESTIONS
1. Define saturated and unsaturated vapour.
Ans. Saturated vapour: At a particular temperature, if maximum amount of vapour is accommodated in a closed space, then that vapour is called saturated vapour.
Unsaturated vapour: If the vapour in a closed space at a particular temperature is less than the highest possible vapour that can be accommodated at that temperature, then that vapour is called unsaturated vapour.
2. What is vapour pressure?
Ans. A liquid can vaporise at any temperature. If a liquid is taken to fill a closed vessel partially, then the liquid vaporises and the empty portion of the vessel is filled up by that vapour. This vapour exerts pressure on the inner surface of the vessel. This pressure is called vapour pressure.
3. What is saturated vapour pressure and unsaturated vapour pressure?
Ans. Saturated vapour pressure: If vapour pressure in a closed space at a specific temperature is equal to the maximum vapour pressure at that temperature, then that vapour pressure is called saturated vapour pressure of that closed space. Unsaturated vapour pressure: If vapour pressure in a closed space at a specific temperature is less than the maximum vapour pressure possible at that temperature, then that vapour pressure is called unsaturated vapour pressure of that closed space.
4. Write down the differences between saturated and unsaturated vapour.
Ans. Differences between saturated and unsaturated. vapours are:
| Saturated vapour |
Unsaturated vapour |
| 1. Maximum amount of vapour present in a closed space at a specific temperature is called saturated vapour. |
1. If a closed space at a specific temperaure contains less amount of vapour than the highest possible vapour at that temperature, then that vapour is called unsaturated vapour. |
| 2. Saturated vapour cannot be converted to unsaturated vapour by increasing temperature or by decreasing pressure. |
2. Unsaturated vapour can be converted to saturated vapour by decreasing temperature or increasing pressure. |
| 3. Saturated vapour remains in equilibrium, when in contact with liquid. |
3. Unsaturated vapour does not remain in equilibrium, when in contact with liquid. |
5. What do you mean by dew point?
Ans. Atmosphere is filled with water vapours almost at all times. If temperature is more, capacity to hold water vapour becomes more. As temperature remains high during the day, capacity to hold water vapour also remains high. So generally during day time, air is not saturated by water vapour present in it. At night, earth’s surface gets colder by radiation of heat, so atmosphere surrounding the surface gets cold. Then capacity of air to hold water vapour also decreases. Thus when temperature of the atmosphere decreases, air at a specific temperature gets saturated by the water vapour present it. This temperature is called dew point. In short, the specific temperature at which a certain volume of air is saturated with water vapour present in it is known as dew point.
6. How does dew originate? What are the essential conditions for formation of dew?
Ans. When temperature of the atmosphere comes down below the dew point, some amount of water vapour of the atmosphere is condensed to form small droplets of water and settle on grass, leaves etc. These droplets are called dew.
Essential conditions for formation of dew: (1) Cloud free clear sky; (2) Calm and still air; (3) Excess of water vapour in atmosphere; (4) Closeness to objects which are bad conductors of heat.
7. Explain how fog is formed.
Ans. If atmospheric temperature of a vast region falls below the dew point due to some reason, then some amount of water vapour present in the atmosphere condenses as deposits on coal particles, dust etc. and floats in the atmosphere. This is called fog. When formed over a lake, it is called mist. As air in an industrial area contains considerable amount of dust, coal particles etc., dense fog is formed in those areas.
8. Explain how cloud is formed.
Ans. Density of water vapour is less than density of air. So, water vapour present in air naturally goes up. As it goes up from the earth’s surface, air pressure decreases. So, volume of water increases due to reduction of air pressure and hence, its temperature decreases. Now as we know that temperature of upper atmosphere remains low, when water vapours come in contact, the temperature of water vapour decreases further. Now if that temperature becomes less than the dew point, then water vapour condenses on dust particles present there. and float around in the form of small water particles and is called cloud.
9. Cloudy sky is not favourable for formation of dews’-explain.
Ans. Since radiated heat from the earth gets reflected back by cloud. For this the earth surface does not get cool sufficiently to help the formation of dew. That is why cloudy sky is not favourable for formation of dews.
10. What do you mean by relative humidity?
Ans. Relative humidity is the ratio of the mass of water vapour present in a particular volume of air to the mass of water vapour required to saturate that volume at a particular temperature. In other words, relative humidity is the ratio of partial pressure of water vapour to the saturated pres- sure of water vapour at a particular temperature.
Now suppose, m is the mass of water vapour present in a specific volume of air at t°C and M is the mass of water vapour to be present at that temperature to make that volume of air saturated.
11. What do you mean by absolute humidity? What is its practical unit?
Ans. Absolute humidity (AH) is defined as the amount of water vapour in grams present per cubic meter of air.
Suppose, water vapour of mass m g is present in a specific amount of air of volume V m³ at temperature t°C.
Practical unit of absolute humidity is g/m³.
12. On a summer day, suppose the temperatures of Delhi and Puri are the same. Which place feels more uncomfortable?
Ans. As Puri is situated on sea shore, value of relative humidity of air in Puri is higher than that in Delhi. Perspiration takes more time to dry up in Puri, as a result an uncomfortable feeling is there, though temperature of these two places are the same.
13. If the temperature of a closed room is increased, is there any change of relative humidity?
Ans. We know that,
If room temperature is increased, capacity of air to hold water vapour increases. Hence, water vapour of greater mass is required (say, M1 where M1 > M) to saturate the air of that room. Since amount of water vapour in the room remains unchanged, hence relative humidity decreases.
14. Is there any change of relative humidity when water is sprinkled in a closed room?
Ans. When water is sprinkled in a closed room, mass of water vapour present in the air of the room increases. Now as the temperature of the room remains unchanged, mass of water vapour necessary to saturate remains unchanged. So, relative humidity of air in the room increases.
15. Is there any change of dew point when water is sprinkled in a closed room?
Ans. When water is sprinkled in a closed room, amount of water vapour in the room increases. Now as the temperature of the room remains unchanged, so in order to make the air of the room aturated, comparatively less reduction of temperature has to be brought about. Hence, dew point increases.
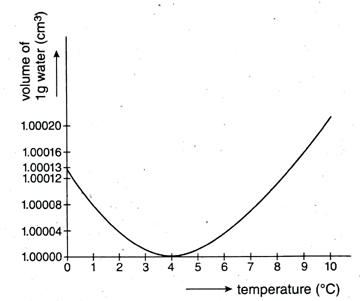
16. What do you mean by anomalous expansion of water? Explain it with a graphical representation of volume and 1+2 temperature.
Ans. In general, if the temperature of a liquid increase, its volume also increases. But in case of water, there is an exception to this rule in a specific range of temperature, i.e., from 0°C to 4°C temperature. If temperature of water is increased from 0°C, it volume decreases and density gradually increases. Density of water is maximum (1g. m³) at 4°C, so the volume of a specific mass is lowest. If temperature is increased after 4°C, volume of water increases and density decreases.
In Fig., change of volume of 1 g of water, when temperature in increased from 0°C to 10°C is shown.
This behaviour of water from 0°C to 4°C temperature is known as anomalous expansion of water.
17. Discuss the influence of anomalous expansion of water on aquatic animals.
Ans. In cold countries, when atmospheric temperature decreases below 0°C, temperature. of the upper surface of lakes, water bodies etc. also comes down gradually. During this time, cold and heavy water from the top of the lake goes down and comparatively warm and light water from the bottom of the lake comes upward. This convection cycle goes on till the temperature of the water at the bottom reach 4°C. At this temperature 4°C, density of water is maximum, i.e., after this temperature, water does not rise any further. Now, temperature of the upper surface of the lakes, water bodies etc. keeps on decreasing. At this temperature, density of water at the top starts decreasing causing water not to go down further.
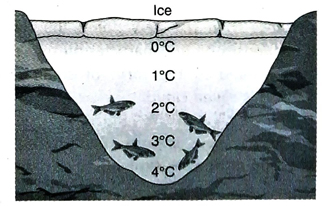
Hence, temperature of the upper surface comes down to 0°C and finally is converted to ice. As ice is lighter than an equal volume of water, it floats on water. Ice is a bad conductor of heat and so rate of transmission of heat from the lower layer is very low and as a result, thickness of ice increases gradually. Some water remains below the ice layer or crust. Temperature of water just below this ice crust is 0°C and temperature increases down the water layers to become 4°C at the bottom. For this reason, though the atmospheric temperature in cold countries comes below 0°C to make the upper surface of lakes frozen, aquatic animals survive easily.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. Hygrometer is used to measure
A. amount of oxygen in air
B. density of air
C. increase of density with temperature
D. amount of water vapour present in the atmosphere
Ans. D
2. If room temperature is equal to the dew point, relative humidity becomes
A. 100%
B. 0%
C. 70%
D. 85%
Ans. A
3. Which of the following statements regarding saturated vapour is true?
A. follows Boyle’s law
B. remains in equilibrium when in contact with the liquid
C. can be made unsaturated by reducing the temperature
D. all of the above
Ans. B
4. Anomalous expansion is seen for
A. mercury
B. kerosene
C. glycerine
D. water
Ans. D
5. In a cold country if the upper surface of a lake is converted into ice, temperature of water at the bottom of the lake becomes
A. 0°C
B. 1°C
C. 2°C
D. 4°C
Ans. D
6. On a particular day, temperature of Kolkata and Delhi are the same. But the levels of relative humidity in air are 80% and 60%, respectively. Which of the following statements is correct?
A. Weather of both the places are equally comfortable
B. Given information regarding weather is incomplete
C. Weather of Kolkata is more comfortable
D. Weather of Delhi is more comfortable
Ans. D
7. The physical quantity determining the amount of water vapour in a definite volume of air is
A. humidity
B. dew point
C. temperature
D. water equivalent
Ans. A
8. If the amount of water vapour increases in air, then
A. density of air increases
B. density of air decreases
C. relative humidity of air decreases
D. dew point of air decreases
Ans. B
9. Formation of fog, takes place generally in
A. summer
B. rainy season
C. winter
D. autumn
Ans. C
10. Formation of dew takes place generally in
A. summer
B. rainy season
C. winter
D. autumn
Ans. D
11. Anomalous expansion of water is observed in the temperature range of
A. 10°C-14°C
B. 4°C-14°C
C. 0°C-4°C
D. 0°C-10°C
Ans. C
12. If relative humidity of air decreases from 80% to 60%, then
A. we feel comfortable
B. uneasiness due to perspiration increases
C. we feel hotter
D. temperature comes down from 80°C to 60°C
Ans. A
13. If water is sprinkled in a closed room, then the room’s
A. relative humidity decreases
B. relative humidity increases
C. dew point decreases
D. dew point remains unchanged
Ans. B
14. If temperature of water is decreased from 4°C to 0°C, then
A. density increases
B. density decreases
C. volume decreases
D. both density and volume decrease
Ans. B
15. If the temperature of water is increased from 4°C to 10°C, then
A. volume of water decreases
B. mass of water decreases
C. volume of water increases
D. mass of water increases
Ans. C
16. Volume expands due to application of heat. On the basis of this information, water behaves normally
A. in the range of 0°C-4°C
B. in the range of 0°C-10°C
C. in the range of 4°C-10°C
D. in the range of 3°C-4°C
Ans. C
17. Temperature of air and its relative humidity are 30°C and 80%, respectively. If 32 mm Hg is the pressure of saturated water vapour at 30°C, then the pressure of water vapour present in air becomes
A. 23.2 mm Hg
B. 24.2 mm Hg
C. 25.6 mm Hg
D. 27.2 mm Hg
Ans. B
18. What is the relative humidity if pressure of water vapour is 27 mm Hg and pressure of saturated water vapour at that temperature is 30 mm Hg?
A. 60%
B. 70%
C. 80%
Answer in brief
1. What is the value of relative humidity, when temperature of the atmosphere is equal to the dew point?
Ans. When temperature of the atmosphere is equal to the dew point, value of relative humidity is 100%.
2. Which liquid shows anomalous expansion?
Ans. Water shows anomalous expansion.
3. At what temperature, density of water is maximum?
Ans. At 4°C or 277 K, density of water is maximum.
4. If the upper surface of a lake in a cold country freezes to ice, what is the temperature of water at the bottom surface?
Ans. If the upper surface of a lake in a cold country freezes to ice, the temperature of water at the bottom surface must be 4°C.
5. If the upper surface of a lake in a cold country freezes to ice, what is the temperature of water just below the ice crust?
Ans. If the upper surface of a lake in a cold country freezes to ice, the temperature of water just below the ice crust must be 0°C.
6. Does saturated vapour follow Charles’ law and Boyle’s law?
Ans. No, saturated vapour does not follow Charles’ law and Boyle’s law.
7. What is the range of temperature in which anomalous expansion of water is seen?
Ans. Anomalous expansion of water is seen in the temperature range of 0°C to 4°C.
8. The volume of a definite mass of water is minimum at what temperature?
Ans. The volume of a definite mass of water is minimum at 4°C.
9. Due to which property of water, aquatic animals survive in spite of atmosphere temperature going down below the freezing point in cold countries?
Ans. This happens due to anomalous expansion of water.
10. A bowl made up of glass is filled with water up to the brim. What is the result if temperature of water is raised and reduced?
Ans. Water spills out for both the rise and fall of temperature.
11. How is unsaturated vapour converted to saturated vapour?
Ans. By increasing the pressure or by decreasing the temperature, unsaturated vapour can be converted to saturated vapour.
12. How is saturated vapour converted to unsaturated vapour?
Ans. By decreasing the pressure or by increasing the temperature, saturated vapour can be converted to unsaturated vapour.
13. Which of the two remains in equilibrium when in contact with the liquid, saturated vapour or unsaturated vapour?
Ans. Saturated vapour remains in equilibrium when in contact with the liquid.
14. What is the required temperature of the atmosphere for formation of fog?
Ans. If temperature of the atmosphere comes down below the dew point, formation of fog takes place.
15. The temperature of a closed room is reduced. Does relative humidity increase or decrease in this case?
Ans. Relative humidity of a closed room increases if the temperature of the room is decreased.
16. Water is sprinkled in a closed room. Is there a change in relative humidity?
Ans. If water is sprinkled in a closed room, amount of water vapour present in the room increases and thus, relative humidity increases.
17. When the upper surface of a lake in a cold country freezes to ice, what is the temperature of water of the bottom surface of the lake?
Ans. Temperature of water = 4°C.
18. Name the instrument used to measure relative humidity of air.
Ans. Hygrometer is used to measure the relative humidity of air.
19. What is dew point?
Ans. The temperature at which a certain volume of air becomes saturated with the water vapour present in it is called dew point of that air.
Fill in t the blanks
1. The capacity of a closed space to contain maximum vapour ………… with increasing temperature.
Ans. Increases
2. ……….. vapour does not remain in equilibrium when in contact with a liquid.
Ans. unsaturated
3. ………… vapour may be converted into ……….. vapour by increasing temperature or by reducing pressure.
Ans. saturated, unsaturated
4. In general, increase of temperature of any liquid ………… its volume but in case of water, there is an ……….. to this rule.
Ans. increases, exception
5. Though Delhi and Puri may have the same temperature on a particular day of summer, we feel warmer in …………..
Ans. Puri
6. When temperature starts ………… volume of water normally reduces to 4°C.
Ans. decreasing
7. When temperature starts ………… anomalous expansion of water is observed upto 4°C.
Ans. increasing
8. …………. of water at 4°C is higher than its density at 3°C.
Ans. density
9. Volume of 1 g of water at 4°C is …………. than the volume of 1 g of water at 2°C. Sadetin
Ans. less
10. ………… vapour exists on the top surface of a liquid in a closed vessel.
Ans. saturated
11. ……….. indicates the amount of water vapour in air.
Ans. humidity
12. Air is ………….. at the dew point.
Ans. saturated
13. The weather of Delhi is more dry compared to that of Kolkata. This means the amount of relative humidity in Delhi is comparatively …………..
Ans. lower
14. ……… is defined as the amount of water vapour in grams present in every cubic metre of air.
Ans. absolute humidity
State whether true or false
1. Saturated vapour is the maximum amount of vapour that can be accomodated in a closed space at a particular temperature.
Ans. True
2. If temperature of the atmosphere goes above the dew point, dew is formed.
Ans. False
3. If temperature of water is increased from 0°C to 4°C, then its volume increases.
Ans. True
4. Clear sky is a favourable condition for the formations of dew.
Ans. True
5. Dew forms only at night.
Ans. True
6. Relative humidity have no unit.
Ans. True