Gujarat Board Textbook Solutions Class 10 Science Chapter 4 Carbon and Its Compounds
Gujarat Board Textbook Solutions Class 10 Science Chapter 4 Carbon and Its Compounds
GSEB Solutions Class 10 Science Chapter 4 Carbon and Its Compounds
Gujarat Board Class 10 Science Carbon and Its Compounds Textbook Questions and Answers
Question 1. Ethane, with the molecular formula C2H6 has –
(a) 6 covalent bonds.
(b) 7 covalent bonds.
(c) 8 covalent bonds.
(d) 9 covalent bonds.
Answer:
(b) 7 covalent bonds.
Question 2. Butanone is a four-carbon compound with the functional group –
(a) carboxylic acid.
(b) aldehyde.
(c) ketone.
(d) alcohol.
Answer:
(c) ketone.
Question 3. While cooking, if the bottom of the vessel is getting blackened on the outside, it means that –
(a) the food is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the fuel is burning completely.
Answer:
(b) The fuel is not burning completely.
Question 4. Explain the nature of the covalent bond using the bond formation in CH3Cl.
Answer:
Bond formation in CH3Cl

Carbon forms four covalent single bonds by sharing four electron pairs with three hydrogen atoms and one chlorine atom. Chlorine being more electronegative adds polar nature to C-Cl bond.
Question 5. Draw the electron dot structure for
(a) ethanoic acid.
(b) H2S
(c) propanone.
(d) F2
Answer:
The electron dot structure are as follows:
(a) Ethanoic acid – CH3COOH

(b) H2S

(c) Propanone

(d) F2

Question 6. What is an homologous series? Explain with an example.
Answer:
It is a group of members of same class of organic compound having similar chemical properties, they have same general formula. They have same functional group, when arranged in the ascending order of molecular mass they differ by 14 a.m.u. or -CH2 group.
Example:

Question 7. How can ethanol and ethanoic acid be differentiated on .the basis of their physical and chemical properties?
Answer:
Physical Properties:

Chemical Properties:

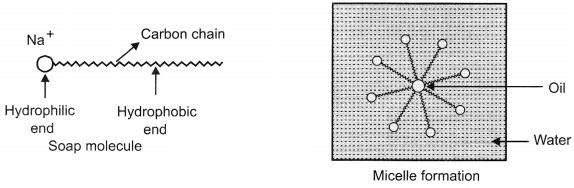
Question 8. Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
Answer:
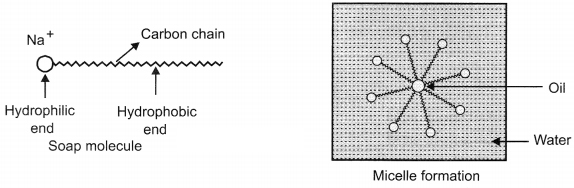
Soap molecules have two ends with different properties. One end is hydrophilic, which dissolves in water and other end is hydrophobic, it dissolves in hydrocarbons. When soap is added to water, the ionic end of soap will form a unique orientation and keep the hydrocarbon tail away from it.
The cluster of molecules is formed in which the hydrophobic tails are in the interior of the cluster and the ionic ends are on the surface of the cluster. Hence, micelle formation takes place. Soap is soluble in ethanol hence the micelle formation will not take place.

Question 9. Why are carbon and its compounds used as fuels for most applications?
Answer:
Carbon and its compounds undergo combustion to produce heat, the amount of heat released can be handled and is adequate to use for most applications.
Question 10. Explain the formation of scum when hard water is treated with soap.
Answer:
Hard water contains salts of calcium and magnesium. When soap molecule comes in contact with these salts it forms a curdy white precipitate (compound insoluble in water) called scum.
Soap + Hard water → scum
Question 11. What change will you observe if you test soap with litmus paper (red and blue)?
Answer:
Soap is alkaline in nature, hence it will turn red litmus into blue, blue litmus will remain blue.
Question 12. What is hydrogenation? What is its industrial application?
Answer:
When unsaturated hydrocarbons (double/triple bond) are reacted with hydrogen in presence of a catalyst like nickel, the hydrogen gets added across the double/triple bond and converts the unsaturated hydrocarbon into saturated hydrocarbon. Such reaction is called addition reaction or hydrogenation.
Example: 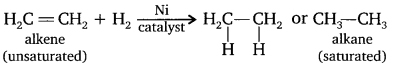
Industrial use: It is used to convert vegetable oil into vanaspati ghee.
![]()
Question 13. Which of the following hydrocarbons undergo addition reactions?
C2H6, C3H8, C3H6, C2H2 and CH4.
Answer:
Addition reaction takes place in unsaturated hydrocarbons.
Hence C3H6 and C2H2 are unsaturated hydrocarbons and will show addition reaction.
Question 14. Give a test that can be used to differentiate chemically between saturated and unsaturated hydrocarbon.
Answer:
Test:
![]()
Therefore, when we add oil to a test tube containing alkaline potassium permanganate solution, the pink colour of the solution disappear. Colour of alkaline potassium permanganate will not disappear in the test tube containing butter.
Question 15. Explain the mechanism of cleaning action of soaps.
Answer:
Soap molecule has two ends, the charged end that gets attracted towards water is called hydrophilic and the long carbon chain that repels water is called hydrophobic tail. When soap is dissolved in water, the carbon chain i.e., hydrophobic end gets attracted towards the oil, dirt and grease. The hydrophilic end stays away from this. The micelle formation takes place.
The tail entangles dirt, oil or grease, if required, the agitation is done. Lot of rinsing is done with water so that water molecules attract charged (Na+) end and carries the soap molecules with dirt attached to it and clean the clothes, utensils, etc.
Gujarat Board Class 10 Science Carbon and Its Compounds Additional Important Questions and Answers
Very Short Answer Type Questions
Question 1. What is a hydrocarbon?
Answer:
It is a compound of hydrogen and carbon.
Question 2. Give different forms in which carbon occurs in nature.
Answer:
Carbon occurs as graphite and diamond in free form and as carbon dioxide, carbonates, etc in combined form.
Question 3. Name two types of hydrocarbon.
Answer:
Hydrocarbon – Saturated and unsaturated.
Question 4. What are covalent bonds?
Answer:
Bond which is formed by sharing of a pair of electrons between two atoms is called covalent bond.
Question 5. What is catenation?
Answer:
Carbon has the unique ability to form bonds with the other atoms of carbon which gives rise to large molecules. This property of self linking is called catenation.
Question 6. Name two alio tropes of carbon.
Answer:
Two allotropes are diamond and graphite.
Question 7. Why covalent compounds have low melting and boiling points?
Answer:
As the bond is formed by sharing of electrons between two atoms. Intermolecular forces are small between the covalent compounds. These bonds break easily.
Question 8. Define oxidising agents.
Answer:
Some substances are capable of adding oxygen to others. These substances are known as oxidising agents.
Example:
alkaline KMnO4 and acidified K2Cr2O7.
Question 9. Give the reaction to show how alcohol is converted into carboxylic acid.
Answer:

Question 10. Identify the compound H

Answer:
Propyne.
Question 11. Name the compound
Answer:
Benzene, C6H6.
Question 12. Give two properties of ethanol.
Answer:
- Liquid at room temperature
- Soluble in H2O in all proportions
Question 13. Give the formula for the functional group of aldehyde.
Answer:
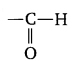
Question 14. What are heteroatoms?
Answer:
An element which replaces one or more hydrogen (H) atoms from hydrocarbon, such that valency of carbon remains satisfied.
Example: CH4 → CH3 – OH
Hence, oxygen is a heteroatom. Complete the following reaction:
Question 15. Complete the following reaction:
 ‘
‘
Answer:

Question 16. Give the full form of IUPAC.
Answer:
IUPAC → International Union of Pure and Applied Chemistry.
Question 17. How can esters be converted into soap?
Answer:
By saponification reaction, by adding/reacting ester with NaOH.
Question 18. How can we convert CH3CH2OH into C2H4?
Answer:
By adding cone, sulphuric acid into it which acts as dehydrating agent and removes water from it.
![]()
Question 19. What is the melting point of acetic acid?
Answer:
M.P = 290 K.
Question 20. Name the given compound
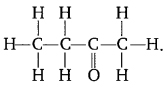 .
.
Answer:
2-Butanone.
Question 21. How can you convert ethene into ethane?
Answer:
By adding hydrogen to ethene in the presence of a catalyst.
Question 22. What is addition reaction? Give one example.
Answer:
The process of adding hydrogen across the double bonds of unsaturated hydrocarbons is called addition reaction.
For example:

Question 23. What is esterification reaction?
Answer:
The reaction in which alcohol reacts with carboxylic acid to produce a new compound called ester is called esterification.
Question 24. Give two uses of methane gas.
Answer:
- It is used as a fuel
- It is the major component of biogas and CNG.
Question 25. What is isomerism?
Answer:
A property in which a compound can exist in different structural formula but its molecular formula remains the same.
Question 26. Why can’t we test hard water with detergents?
Answer:
Detergents form lather with both hard and soft water hence we cannot distinguish between them.
Question 27. What is hydrophilic?
Answer:
The substance showing attraction towards water is called hydrophilic.
Question 28. Name the second member of alkyne series.
Answer:
Propyne
Question 29. Give the names of the functional group
(i) -CRO
(ii) ![]()
Answer:
(i) -CHO → Aldehyde
(ii) ![]() → Ketone
→ Ketone
Question 30. The structural formula of an ester is

Name the alcohol and the acid from which it would have been formed.
Answer:
Alcohol is C2HOH, ethanol acid is H3C-H2C-COOH, propanoic acid.
Question 31. Give the IUPAC name of acetic acid and propyl alcohol.
Answer:
Acetic acid – Ethanoic acid; Propyl alcohol – Propanol
Question 32. What will happen to the litmus solution in carboxylic acid?
Answer:
Red litmus remains the same but blue litmus changes to red.
Question 33. Give the electron dot structure of CH3Cl and C2H2.
Answer:

Question 34. Draw the electron dot structure of N2 and NH3.
Answer:

Question 35. What happens when ethanol burns in air?
Answer:
Ethanol burns to form carbon dioxide and water.
Question 36. Give the TUPAC name and write the functional group present in vinegar.
Answer:
IUPAC name of vinegar is acetic acid CHCOOH.
Functional group – COOK.
Question 37. A compound has a molecular formula C2H6O. It is used as a fuel. Name the compound and name its functional group.
Answer:
C2H6O is an alcohol. i.e. ethanol C2H5OH. Its functional group is -OH.
Short Answer Type Questions
Question 1. What is the reactive site in the given hydrocarbon? Write its name.
H3C-CH2-CH=CH-CH3
Answer:
The reactive site is at a place where double bond is present. Name of the compound is 2-pentene.
Question 2. What is the difference in the number of carbon and hydrogen atoms between two successive members of a homologous series? Also give the difference in their atomic masses.
Answer:
The difference is of 1 carbon and two hydrogen atoms i.e., -CH2 and mass difference is 14 a.m.u.
Question 3. Name the peculiar/specific chemical property exclusive in case of saturated hydrocarbons and unsaturated hydrocarbons.
Answer:
Saturated hydrocarbons show substitution reaction in which hydrogen atom gets substituted by other elements or atoms. Unsaturated hydrocarbons show addition reaction, in which hydrogen atom gets added across the double bond or triple bond of the compound.
Question 4. Why acetic acid is called glacial acetic acid?
Answer:
Acetic acid has very low melting point i.e. 290 K, hence it freezes during winters in cold countries. So it is called glacial acetic acid.
Question 5. Why does carbon forms large number of compounds?
Answer:
Carbon forms large number of compounds because of tetravalency and catenation property.
Tetravalency – Carbon has valency 4, to attain noble gas configuration carbon share its valence electrons with other elements- like hydrogen, chlorine, etc.
Catenation – Carbon also shows the property of self-linking in which it forms long, branched or cyclic chains to form large number of compounds.
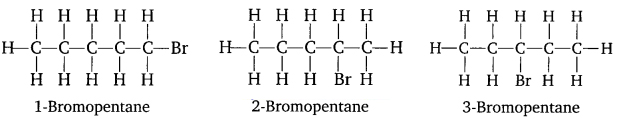
Question 6. Write the structural formula for bromopentane and ethanoic acid.
Answer:

Question 7. How does ethanoic acid react with carbonates and hydrogen carbonates? Show it with the equation.
Answer:
Ethanoic acid reacts with carbonates and hydrocarbonates to form salt, CO2 and H2O. The salt formed is sodium acetate.
2CH3COOH + Na2CO3 → 2CH3COONa + H2O + CO2
CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
Question 8. Draw the structures of two isomers of butane.
Answer:
Butane C4H10

Question 9. A student bums a hydrocarbon in air and obtains sooty flame. Give two reasons for this observation.
Answer:
Sooty flame could be obtained due to:
- Incomplete combustion of saturated hydrocarbons.
- Combustion of unsaturated hydrocarbon.
Question 10. Differentiate between saturated and unsaturated hydrocarbons. Give one example for each.
Answer:
Saturated hydrocarbon:
- It consist of single bond between carbon-atoms.
- It burns with blue flame.
- Show substitution reaction
- Less reactive
- Example: CH4 Methane, C2H6 Ethane
Unsaturated hydrocarbon:
- It consists of double or triple bond between carbon-atoms.
- It burns with sooty flame.
- Show addition reaction.
- More reactive
- Example: H2C=CH2Ethene, HC ≡CH Ethyne
Question 11. Write the general formula for each of the following hydrocarbons and give one example for each.
(i) Alkene
(ii)Alkyne
Answer:
(i) Alkene C2H2n
Example:
C2H4 ethene
(ii) Alkyne CnH2n-2
Example:
C2H2 ethyne
Question 12. Name the functional groups of the following:
(a) CH3-Cl
(b) 
(c) 
(d) C2H5OH
Answer:
(a) Chloro (Halogen)
(b) Carboxylic acid
(c) Ketone
(d) Alcohol
Question 13. Explain substitution reaction with example.
Answer:
The reaction of saturated hydrocarbon with chlorine in which hydrogen atom slowly gets substituted with chlorine atom is called substitution reaction.

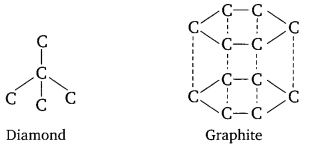
Question 14. Diamond and graphite show different physical properties although they are made up of carbon and shows same chemical properties. What is this property called?
Answer:
This property is allotropy. The physical properties are different because the carbon-carbon bonding in both the cases varies. In diamond, one carbon atom is bonded with four other carbon atoms with strong. covalent bond so it is hard, while in case of graphite each carbon forms three strong bonds with other two carbon atoms and forms layers of hexagonal rings which slide over each other, so it is soft.
Question 15. What is denatured alcohol?
Answer:
When ethanol is mixed with methanol or some poisonous substances such as copper sulphate, pyridine which makes it unfit for drinking such alcohol is called denatured alcohol.
Question 16. What is esterification and give its uses?
Answer:
It is the reaction in which esters are formed by reacting carboxylic acid with alcohol in the presence of concentrated sulphuric acid.

Uses:
- It is used as flavour in ice-cream and sweets.
- It is a sweet smelling substance so used in perfumes.
Question 17. Give difference between soap and detergent.
Answer:
Soap:
- Soaps are the sodium salts of the long chain carboxylic acids.
- Soaps are not suitable for washing with hard water as it forms insoluble scum.
- Soaps are prepared from fats or vegetable oils.
- Bioderadable
Detergent:
- Detergents are generally sodium salts of long chain suiphonic acids or long carbon chain ammonium salts of chloride or bromide ions.
- Detergents do not form insoluble scum with hard water.
- Detergents are not prepared from fats or vegetable oils.
- Non-biodegradable.
Question 18. Differentiate between ethanol and ethanoic acid on basis of the following test:
(i) Blue litmus test
(ii) Reaction with sodium bicarbonate
(iii) Sodium metal test
Answer:

Question 19. Giving chemical equations of the reactions write what happens when
(i) Ethanol is heated with excess of concentrated sulphuric acid at 443 K.
(ii) Ethanoic acid reacts with ethanol in presence of an acid.
(iii) Ester with molecular formula CH3COOC2H5 reacts with sodium hydroxide.
Answer:
(i) 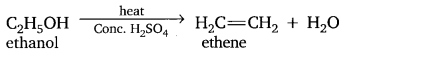
(ii) ![]()
(iii) CH3COOC2H5 + NaOH → C2H5OH + CH3COONa
Question 20. How can you obtain the following from pure ethanol:
(i) Ethene
(ii) Ethanoic acid
(iii) Ester?
Answer:
(i) Ethene: Ethanol when heated with excess of concentrated sulphuric acid will form ethene.

(ii) Ethanoic acid: On oxidation of ethanol with an oxidising agent like alkaline KMnO4 or acidified K2Cr2O7, ethanoic acid is formed.

(iii) Ester: To get esters, ethanol is reacted with any carboxylic acid in presence of acid.

Question 21. Write the chemical equations for the following reactions:
(i) Conversion of oils into fats
(ii) Oxidation of ethanol
(iii) Ethanoic acid with sodium hydroxide.
Answer:
(i) 
(ii) Oxidation of ethanol

(iii) 
Question 22. An organic compound ‘X’ which is also called antifreeze mixture when mixed with water has the molecular formula C2H6O. ‘X’ on oxidation gives a compound Y which gives effervescence with a baking soda solution. What can X and Y be? Write their structural formula.
Answer:
X is ethanol, (C2H5OH)
Y is ethanoic acid (CH3COOH)
Structural formula:

Question 23. Write the structures of isomers of hexane.
Answer:

Question 24. Complete and balance the following equations:
(a) CH3CH2OH + O2→
(b) Na + CH3CH2OH→
(C) ![]()
Answer:
(a) CH3CH2OH + 3O2 → 2CO2 + 3H2O + heat + light
(b) 2Na + 2CH3CH2OH → 2CH3CH2ONa + H2
(C) ![]()
Question 25. Give two uses of ethanol and one harmful effect of it.
Answer:
Ethanol is a good solvent so it is used in making medicines such as tincture iodine, cough syrups and many tonics. Ethanol is also used in making alcoholic drinks.
Harmful effects: Intake of even small amount of ethanol can be lethal. Long-term use or consumption can lead to severe health problems.
Question 26. (a) Why are covalent compounds generally poor conductors of electricity?
(b) Name the following compound:

(c) Name the gases evolved when ethanoic acid is added to sodium carbonate. How would you prove the presence of this gas?
Answer:
(a) Covalent compounds do not form ions which can conduct electricity.
(b) Propanone
(c) Ethanoic acid reacts with sodium carbonate to produce carbon dioxide gas. To prove the presence of this gas allow it to pass through lime water (freshly prepared). It turns lime water milky,
Equation:

Long Answer Type Questions
Question 1. An organic compound ‘A’ is widely used as a preservative in pickles and has a , molecular formula C2H4O2. This compound reacts with ethanol to form a sweet, smelling compound ‘B’.
(a) Identify the compound A’.
(b) Write the chemical equation for its reaction with ethanol to form compound ‘B’.
(c) How can we get compound A’ and ‘B’?
(d) Which gas is obtained when compound A reacts with washing soda? Give the equation.
(e) Write an equation to obtain A back from ‘B’.
Answer:
(a) ‘A’ is CH3COOH, acetic acid.
(b) ![]()
(c) By oxidation of ethanol we get A (acetic acid)

(d) A + washing soda → CO2 gas is produced
2CH3COOH + Na3CO3 → 2CH3COONa + H2O + CO2
(e) Saponification

Question 2. Identify the compound A, B, C, D, and E in the following reaction:
(a) ![]()
(b) ![]()
(c) B + NaOH → C2H5OH + C
(d) D + Na2CO3 → CH3COONa + E + H2O
(e) E + Ca(OH)2 F + H2O
Answer:
(a) A = Alkaline KMnO4 or acidified K2Cr2O7
(b) B = CH3COOC2H5
(c) C = CH3COONa, B = CH3COOC2H5
(d) D = CH3COOH; E = CO2
(e) E = CO2; F = CaCO3
Question 3. What are soaps? Explain the mechanism of the cleansing action of soaps? Soaps form scum with hard water. Explain why? How this problem is overcome by use of detergents?
Answer:
Soaps are sodium salts of fatty acids. It is biodegradable and shows cleansing action by removing dirt.
Mechanism of cleansing action:
Soap molecule has two ends, the charged end that gets attracted towards water is called hydrophilic and the long carbon chain that repels water is called hydrophobic tail. When soap is dissolved in water, the carbon chain i.e., hydrophobic end gets attracted towards the oil, dirt and grease. The hydrophilic end stays away from this. The micelle formation takes place.
The tail entangles dirt, oil or grease, if required, the agitation is done. Lot of rinsing is done with water so that water molecules attract charged(Na+) end and carries the soap molecules with dirt attached to it and clean the clothes, utensils, etc.
In case of detergents, the salts present in hard water does not react with the molecules of detergent to form insoluble scum, but the molecules of detergent remain as it is and helps in the cleansing action.
Scum formation:
Hard water contains salts of calcium and magnesium. When soap molecule comes in contact with these salts it forms a curdy white precipitate (compound insoluble in water) called scum.
Soap + Hard water → scum
Question 4.
(a) What do you mean by allotropy?
(b) What is isomerism?
(c) Give one example of homologus series, give two properties of it.
(d) What is the full form of IUPAC?
Answer:
(a) Allotropy: It is the property of an element in which element show same chemical properties but different physical properties, due to difference in the bonding of atoms.
Example: Diamond and graphite are having same chemical properties but they look/appear to be physically different as the bonding in both
differs.
(b) Isomerism: It is the property of hydrocarbons which show same molecular formula but exhibits different structural formulae.
Example: Butane

Both of them show different properties.
(c) Homologous series: When the members of a hydrocarbon family obey same general formula they are said to be in homologous series. In this series, members are arranged in increasing order of their molecular masses:
Example: Alkane – CnH2n+2
CH4 — Methane
C2H6 — Ethane
C3H8 — Propane
C4H10 — Butane
Properties:
- The difference between two consecutive members of homologous series is of —CH2 and mass 14 a.m.u.
- They all show same chemical properties and slight gradation in their physical properties.
(d) IUPAC: International Union of Pure and Applied Chemistry.
Question 5.
(a) What are hydrocarbons?
(b) Give difference between saturated and unsaturated hydrocarbons.
(c) Why does carbon form large number of compounds?
Answer:
(a) Hydrocarbons – A compound of carbon and hydrogen.
(b) 
(c) Carbon forms large number of compounds due to
- Catenation – Self linking property which leads to long straight chains, branched chains and cyclic chains.
- Isomerism – Compound of carbon can exist in more than one structural formula but has same molecular formula.
- Tetravalency – To acquire noble gas configuration, carbon shares its outer electrons with other elements, thus form covalent bond with other elements
Questions On High Order Thinking Skills (Hots)
Question 1.
A, B, C are members of homologous series and their melting points are -183°C, -138°C, 130°C respectively. Among these
- Which member will have least number of carbon atoms?
- Which member will have maximum number of carbon atoms?
Answer:
- A will have least number of carbon atoms.
- C will have maximum number of carbon atoms.
Question 2.
A hydrocarbon compound A is active ingredient of wine and cough syrup. A on oxidation with acidified K2Cr2O7 forms compound B. Identify the compound A and B and write the chemical equations involved.
Answer:
A is ethanol, C2H5OH
B is ethanoic acid, CH3COOH
Equation :
![]()
Question 3.
Write an activity to show the acidic nature of ethanol. Give the chemical equation of the reaction taking place.
Answer:
Take ethanol in a test tube and drop a small piece of sodium about the size of a grain of rice into it. The reaction evolves a colourless gas which is hydrogen.
Hydrogen gas can be tested by bringing a burning splinter/matchstick near the mouth of the test tube, it burns with the popping sound.
This activity proves that ethanol like other acids release H2 gas
2Na + 2CH3CH2OH → 2CH3CH2ONa + H2
Question 4.
A compound ‘X’ has molecular formula C2H6O is saturated hydrocarbons and is a very good solvent. How can you convert it into unsaturated hydrocarbon? Identify X and show its conversion with the help of equation.
Answer:
‘X’ is CH3-CH2OH ethanol. It can be made unsaturated by heating it with cone. H2SO4 which is a dehydrating agent and removes water from it, thereby forming ethene.

Question 5.
Take about 20 mL of castor oil in a beaker. Add 30 mL of 20% sodium hydroxide solution. Heat the mixture with continuous stirring for a few minutes till the mixture thickens. Add 5-10 g of common salt to this. Stir the mixture well, allow it to cool, soaps is obtained.
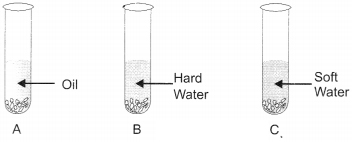
(a) Why do we use common salt to make soap?
(b) What will happen if you will add the above made soap solutions to the following test tubes A, B, and C.
(c) Can we use potassium hydroxide instead of sodium hydroxide.
Answer:
(a) Salt: NaCl is added while making soap, because it will cause precipitation of soap in solid form.
(b) In test tube A: Soap + Oil → Lather/foam is formed. Carbon chain of carboxylic salt dissolves in oil.
In test tube B: Soap + Hard water →Insoluble compound called scum is formed.
In test tube C: Soap + soft water → Froth is formed.
(c) Yes, we can use potassium hydroxide to prepare soap.
Practical Based Questions Solved (Solved)
Question 1.
Give one commercial use and one domestic use of acetic acid.
Answer:
Commercial use: it is used to make ester.
Domestic use: it is used to make vinegar which is used as an ingredient in kitchen for many cooking dishes.
Question 2.
A student wants to find the freezing temperature of acetic acid. List the materials required to do this test in the lab.
Answer:
The materials required are test tube, acetic acid, thermometer and a beaker containing ice.
Question 3.
Draw the observation table for the above experiment and write the prediction for the same.
Answer:
The observation table is :

Prediction: The acetic acid should freeze at the temperature below 17 degrees as it has the freezing temperature of 16.6 degree C.
Question 4.
On adding sodium bicarbonate the acetic acid releases carbon dioxide gas, suggest two ways of testing this gas in the lab.
Answer:
- Allow the gas obtained to pass through freshly prepared lime water, if it turns milky the gas is carbon dioxide.
- Keep a burning candle near the mouth of the delivery tube through which the gas is coming out, the burning candle will extinguish.
Question 5.
Why is acetic acid called a weak acid?
Answer:
The pH of acid is slightly higher as it dissociated partially to form H+ and CO– ions.
Question 6.
Soap is prepared in the lab by the reaction called saponification. Give one example of this reaction.
Answer:
The reaction of (organic acid) fatty acids with sodium hydroxide gives soap and glycerol. Soap is the sodium salt of fatty acids.
Question 7.
How is soap helpful in cleaning action?
Answer:
Soap molecules form micelle when added to a bucket containing dirty/oily cloth soaked in water. The hydrophobic tail attracts the oil and the hydrophilic part of the soap molecule attract the water and hence on rinsing removes the dirt.
Question 8.
State the conditions for obtaining the best cleansing action of soap.
Answer:
The water used should be soft water and not hard water. The soap should have more of soap molecules and less of impurities or adulterants in it.
Question 9.
What is the environmental impact of excess use of detergents?
Answer:
The detergents are non biodegradable and pollutes water thereby killing many aquatic life creatures.
Question 10.
How can you prepare a hard water in the lab and show the ineffective action of soap in it.
Answer:
To make hard water in lab take normal water and dissolve some calcium and magnesium salts of sulphates and phosphates in it. To show the ineffective action of soap with this water just add little sample of soap solution to it, the sample will not form any froth and the curdy white precipitate will be formed.
Gujarat Board Class 10 Science Carbon and Its Compounds InText Questions and Answers
Question 1. What would be the electron dot structure of carbon dioxide which has the formula CO2?
Answer:
The electron dot structure of CO2 is
Question 2. What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur?
Answer:

Question 3. How many structural isomers can you draw for pentane?
Answer:
Three structural isomers can be drawn from pentane.
Pentane : C5H12
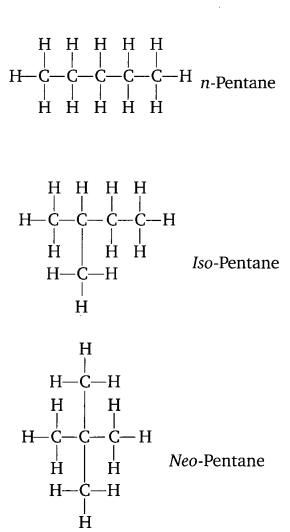
Question 4. What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Answer:
Carbon form large number of compounds due to the following properties:
(a) Catenation → Carbon shows the property of catenation that is the ability to form bonds with other carbon atoms forming long chains both branched and unbranched chains, and even rings.
(b) Tetravalency → Carbon has valency 4, it is capable of bonding with 4 other carbon atoms or atoms of other monovalent elements, giving rise to compounds with specific properties depending on the elements present in the compound.
(c) Isomerism → Carbon compounds show the property of isomerism that is compounds having same molecular formula but different structural formula.
Question 5. What would be the formula and electron dot structure of cyclopentane?
Answer:
The formula of cyclopentane is C5H10.
The electron dot structure is

Question 6. Draw the structures for the following compounds.
(i) Ethanoic acid
(ii) Bromopentane
(iii) Butanone
(iv) Hexanal
Are structural isomers possible for brom opentane?
Answer:
(i) Ethanoic acid CH3COOH

(ii) Bromopentane C5H11Br

(iiii) Butanone C2H5COCH

(iv) Hexanal [C5H11CHO]
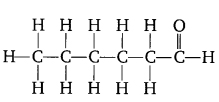
Isomers of bromopentane:
Question 7. How would you name the following compounds:
(i) CH3-CH2-Br
(ii) 
(iii) 
Answer:
(i) Bromoethane
(ii) Methanal
(iii) Hex-l-yne
Question 8. Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
Answer:
Conversion of ethanol to ethanoic acid is an oxidation reaction because oxygen is added to ethanol and hydrogen is removed to convert it to ethanoic acid.

In the above reaction alk. KMnO4/acidified K2Cr2O7 add oxygen to ethanol hence they are called oxidising agent.
Question 9. A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
Answer:
If air is used, incomplete combustion will take place giving a sooty flame and less heat is produced. When pure oxygen is used ethyne bums completely producing large amount of heat and blue flame. This heat is sufficient for a metal to melt and welding is done.
Question 10. How would you distinguish experimentally between an alcohol and a carboxylic acid?
Answer:
(a) Acid test: Reaction with carbonates/hydrogen carbonates. Take samples of alcohol and carboxylic acid in 2 test tubes, and add sodium carbonate or sodium bicarbonate solution to each. The compound which will produce brisk effervescence of CO2 gas will be acid.
(b) Alcohol test: Take small amount of ethanol and ethanoic acid in test tube A and B. Add 5% solution of alkaline potassium permanganate drop by drop to this solution and warm the test tube. The colour of potassium permanganate will disappear in test tube containing alcohol.
Question 11. What are oxidising agents?
Answer:
The compounds which add oxygen to other substance are known as oxidising agent. For example, alkaline potassium permanganate solution and acidified potassium dichromate, both can convert alcohol into carboxylic acid, i.e., ethanoic acid.

Question 12. Would you be able to check if water is hard by using detergent?
Answer:
No, because detergent forms lather in both, hard and soft water.
Question 13. People use a variety of methods to wash clothes. Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat it with a paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is agitation necessary to get clean clothes?
Answer:
Soap lowers the surface tension of water. The long chain non-ionic hydrocarbon group in soap gets attached to the oil or grease droplets and loosens them from the fibres of cloth. However, this loosening is insufficient to remove the grease with dirt completely. Hence, the clothes are agitated to remove the grease droplets completely.
In-Text Activities Solved
Activity 4.1
Answer:
List of ten things used or consumed daily

(C) → indicates carbon
Most substances contain carbon in it.
(a) 
differ By CH2
Mass of Ch3OH
Mass of C2H5OH
(12 x 2) + (1 x 5) + 16 + 1 = 46 u
Difference → 46 – 32 = 14 u
(b) 
differ by CH2
Mass of C2H5OH
(12 x 2) + (1 x 5) + 16 + 1 = 46 u
Mass of C3H7OH
(12 x 3) + (1 x 7) + 16 + 1 = 60 u
Difference → 60 – 46 = 14 u
(c) 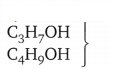
differ by CH2
Mass of C3H7OH
(12 x 3) + (1 x 7) + 16 + 1 = 60 u
Mass of C4H9OH
(12 x 4) + (1 x 9) + 16 + 1 = 74 u
Difference → 74 – 60 =14 u
All 3 groups given above show a similarity → Two consecutive members of homologous series differ by CH2 group and mass 14 u.
CH3OH, C2H5OH, C3H7OH, C4H9OH form a homologous series.
Activity 4.2
Answer:
Homologous Series:

Activity 4.3
Answer:
Heating of different carbon compounds, observing the flame and smoke.

Alcohol and acetone burns with non-sooty flame-complete combustion takes place. Camphor, naphthalene burns with sooty flame-(unsaturated hydrocarbons) incomplete combustion takes place.
Activity 4.4
Answer:
Bunsen burner is used to study the different types of flame by adjusting the holes at the base of the burner.
- When hole is closed – Yellow, sooty flame is formed, black deposits of carbon is obtained on spoon when placed above the flame.
- When the hole is open – Blue flame is formed, no black deposits of carbon is obtained on spoon when placed above the flame.
Activity 4.5
Answer:
Take 3 mL of ethanol in a test tube and warm it gently in a water bath. Add 5% solution of alkaline potassium permanganate drop by drop to the warmed ethanol solution. The colour of KMnO4 slowly fades and disappears completely. When excess of KMnO4 is added, the colour will not disappear as alcohol gets oxidised to form carboxylic acid.
In this case ethanol gets oxidised to form ethanoic acid due to KMnO4 but the excess KMnO4 will not decolourise.

Activity 4.6
Answer:
Take a test tube with ethanol in it and drop a small piece of sodium metal in it. The reaction takes place and hydrogen gas is evolved. To test the presence of hydrogen gas, bring a burning match stick near the mouth of the test tube, it will burn with pop sound.
![]()
Activity 4.7
Answer:

Activity 4.8
Answer:
Take 1 mL ethanol and 1 mL glacial acetic acid along with a few drops of concentrated sulphuric acid in a test tube. Warm in a water-bath for at least five minutes. Pour into a beaker containing 20-50 mL of water.
1. Pleasant fruity smelling compound is obtained called ester.
2. Such a chemical reaction is called esterification.

Activity 4.9
Answer:
Take a spatula full of sodium carbonate in a test tube and add 2 mL of dilute ethanoic acid. Pass the gas produced through freshly prepared lime water. Repeat the above procedure with sodium hydrogen carbonate.
Following reactions take place
2CH3COOH + Na2CO3 → 2CH3COONa + H2O + CO2
CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
The brisk effervescence of CO2 gas is obtained which when passed through lime water turns it milky.

Activity 4.10
Answer:
Take about 10 mL of water each in two test tubes. Add a drop of oil (cooking oil) to both the test tubes and label them as A and B. To test tube B, add a few drops of soap solution. Now, shake both the test tubes vigorously for the same period of time and then leave it undisturbed for some time. The separated layers of oil and water are visible immediately in test tube A but it takes some time to separate the layers in test tube B.
Activity 4.11
Answer:
Take about 10 mL of distilled water and 10 mL of hard water in separate test tubes. Add a couple of drops of soap solution to both. Shake the test tubes vigorously for an equal period of time. The test tube which contains distilled water produces foam and the test tube with hard water forms curdy white precipitate.
Activity 4.12
Answer:
Take two test tubes with 10 mL of hard water in each. Add five drops of soap solution to one and five drops of detergent solution to the other. Shake both the test tubes for the same period.
- Solution of hard water and soap forms curdy white precipitate.
- The hard water and detergent forms foam.
Follow on Facebook page – Click Here
Google News join in – Click Here
Read More Asia News – Click Here
Read More Sports News – Click Here
Read More Crypto News – Click Here