PSEB Solutions for Class 9 Science Chapter 2 Is Matter Around Us Pure
PSEB Solutions for Class 9 Science Chapter 2 Is Matter Around Us Pure
PSEB 9th Class Science Solutions Chapter 2 Is Matter Around Us Pure
→ A pure substance consists of only one type of particle.
→ Mixtures are constituted by more than one kind of pure form of matter known as a substance.
→ The mixture is obtained by mixing one or more pure elements and/or compounds.
→ A substance cannot be separated into other kinds of matter by any known physical process.
→ Whatever the source of the substance may be, it will always have the same characteristic properties.
→ Homogeneous mixtures may have different separate components.
→ Heterogeneous mixtures can be separated into their respective constituents by simple physical methods.
→ A solution is a homogeneous mixture of two or more substances.
→ Alloy is a solid solution and the air is a gaseous solution.
→ The component of a solution which is generally in small amounts and is dissolved into another component is called the solute.
→ The component of a solution that dissolves the other component is called the solvent.
→ This component is generally present in large amounts.
→ Air is a mixture of gas. A solution of sugar in water is solid in a liquid solution. A solution of iodine in alcohol is known as ‘tincture of iodine.
→ Aerated drinks are gas in liquid solutions.
→ A solution is a homogeneous mixture.
→ The particles of the solution are smaller than 1 nm (10-9 m) in diameter.
→ Depending upon the amount of solute present in the solution, it can be called a dilute, concentrated or saturated solution.
→ The different substances have different solubilities in the given solvent at the same temperature.
→ Suspension is a heterogeneous mixture and the particles of a suspension can be seen with a naked eye.
→ In the suspension, the solute particles do not dissolve but remain suspended throughout the bulk of the medium.
→ The suspended particles have sizes of more than 100 nm.
→ The particles of a colloid are uniformly spread throughout the solution.
→ The scattering of a beam of light is called the Tyndall effect.
→ The size of colloidal particles lies between 1 to 100 nm and they can’t be seen with the naked eye.
→ The colloidal particles cannot be separated from the mixture by the process of filtration but can be separated by ultracentrifugation.
→ The volatile component of a solution (solvent) can be separated from the non-volatile component (solute) by the method of evaporation.
→ The cream is separated from milk by a centrifugal machine.
→ Ammonium chloride, camphor, naphthalene, and anthracene can be separated by sublimation.
→ The process of separation of coloured components of a mixture is known as chromatography.
→ The crystallization method is used to purify solids.
→ Crystallization technique is better than simple evaporation technique.
→ Colour, hardness, rigidity, fluidity, density, melting point, boiling point, etc. are the physical properties.
→ Chemical change brings a change in the chemical properties of a matter and we get new substances.
→ A chemical change is also called a chemical reaction.
→ Robert Boyle was the first scientist to use the term element in 1661.
→ An element is a basic form of matter that cannot be broken down into simple substances by chemical reactions.
→ Elements can be normally classified into metals, non-metals, and metalloids.
→ Mercury is the only metal that is liquid at room temperature.
→ Metalloids show the properties of metals as well as non-metals.
→ Pure substances can be elements.
→ The process of separation of components of a mixture containing two miscible liquids that boil without decomposition and have sufficient difference in their boiling points.
→ The different gases in the air and different components of petroleum can be separated by fractional distillation.
→ Mixtures are of two types:
- Homogeneous mixtures
- Heterogeneous mixtures
→ Pure Substance: It is a material containing particles of only one kind having a definite set of properties. Pure substances include elements and compounds.
→ An Element is a pure substance that is made up of only one kind of particle called atoms.
→ It can neither be built up nor broken down into two or simpler substances by any known physical or chemical methods, e.g., copper, silver, etc.
→ A compound is a pure substance that is obtained by the chemical combination of two or more elements in a fixed ratio by mass, e.g., water, ammonia, etc.
→ Mixture: It is a material obtained by mixing two or more substances in any proportion without any chemical change taking place.
→ The solution is a homogeneous mixture of two or more substances whose composition can be changed within certain fixed limits.
→ A binary solution is a solution having two components.
→ The solute is the minor component of a solution whereas solvent is the major component of a solution.
→ The concentration of a solution is the amount of solute present per unit volume or per unit mean of solvent or solution.
→ A saturated solution is one that does not dissolve any more of the solute at a given temperature and pressure.
→ Colloids are heterogeneous mixtures in which the particles have a size of more than 100 nm.
→ These particles are called colloidal particles and constitute the dispersed phase whereas the medium in which colloidal particles are dispersed constitutes the dispersion medium.
→ Suspensions: Materials that are insoluble in a solvent and have particles that are visible to naked eyes form suspensions.
→ Physical Change: It is a temporary change in which only the physical properties of substances change and can be reversed.
→ Chemical Change: It is a permanent change in which the chemical properties of substances change and there is a change in composition and cannot be reversed.
→ Filtration: The process of separation of an insoluble solid component of a mixture from a liquid component is called filtration.
→ Evaporation: It is the slow process of conversion of liquid into a gaseous state (vapour) at a temperature below its boiling point.
→ Distillation: It is the process of conversion of liquid into a gaseous state by heating it to the boiling point and condensing the vapour to get pure liquid.
→ Fractional distillation: It is the process of separating two miscible liquids having different boiling points by distillation using a fractionating column.
→ Chromatography: It is the process of separation of dissolved components of a mixture by adsorbing on a suitable substance (called adsorbent).
PSEB 9th Class Science Important Questions Chapter 2 Is Matter Around Us Pure
Long Answer Type Questions:
Question 1.
(a) Define mixture. Give examples.
(b) What are the types of mixtures?
Answer:
(a) Mixture. It is a product obtained by mixing two or more substances (elements and / or compounds) in any proportion. For example,
- Air is a mixutre of nitrogen, oxygen, carbon dioxide, water vapour etc.
- Smoke is a mixture of carbon particles and air.
- Gun Powder is a mixture of nitre, sulphur and charcoal.
- Brass is a mixture of copper and zinc.
(b) Types of mixtures. Mixtures are of two types:
1. Homogeneous mixtures and
2. Heterogeneous mixtures.
1. Homogeneous mixtures: A mixture in which the different constitutents are mixed uniformly and has the uniform composition throughout is called homogeneous mixture, e.g. Brass is a homogeneous mixture of copper and zinc, an aqueous solution of sodium chloride is a homogeneous mixture of sodium chloride and water.
2. Heterogeneous mixtures: A mixture in which the different constitutents are not mixed uniformly is called heterogeneous mixture, e.g. soil is a heterogeneous mixture, a mixture of common salt, sand and sulphur is a heterogeneous mixture.
Question 2.
Give important characteristics of a mixture.
Answer:
The important characteristics of a mixure are:
- In a mixture, the components, elements or compounds are present in no fixed ratio.
- A mixture is formed from its components as a result of physical change.
- The properties of a mixture lie between those of its constituents.
- A mixture may be homogeneous or heterogeneous.
- The constituents of a mixture can be separated by physical methods only.
- The formation of a mixture from its constituents does not involve any energy change.
Question 3.
What are the various types of binary solutions based upon the physical states
of solute and solvent?
Answer:
These are of nine types:
| Solute | Solvent | Type of solution | Example |
| 1. Solid | Solid | Solution of solid in solid | Brass |
| 2. Solid | Liquid | Solution of solid in liquid | Sugar in water |
| 3. Solid | Gas | Solution of solid in gas | Iodine in air |
| 4. Liquid | Solid | Solution of liquid in solid | CuS04. 5H20 |
| 5. Liquid | Liquid | Solution of liquid in liquid | Ethyl alcohol in water |
| 6. Liquid | Gas | Solution of liquid in gas | Moisture in air |
| 7. Gas | Solid | Solution of gas in solid | Hydrogen in palladium |
| 8. Gas | Liquid | Solution of gas in liquid | Carbon dioxide dissolved in water |
| 9. Gas | Gas | Solution of gas in gas | Air |
Question 4.
State briefly how would you separate or name the process use to separate:
- Common salt from a solution of common salt and water.
- Alcohol from a mixture of alcohol and water.
- Sulphur from a mixture of carbon particles and powdered roll sulphur.
- The coloured dyes in black ink.
Answer:
- The solution containing common salt in water is concentrated and is then cooled. The crystals of common salt are formed which can be separated by filtration.
- Alcohol can be separated from water by fractional distillation as the two liquids differ much in their boiling points.
- The mixture is treated with carbon disulphide which dissolves powdered sulphur in it. It is filtered to separate carbon particles. The filtrate upon concentration and cooling gives crystals of sulphur which can be separated by the process of filtration.
- The coloured dyes in black ink can be separated by the process of paper chromatography.
Question 5.
Give important differences between a compound and a mixture.
Answer:
| Compound | Mixture |
| 1. A compound is formed from its constituent elements as a result of chemical reaction.
2. A compound is always homogeneous in nature. 3. In a compound the elements are present in a fixed ratio by weight. 4. The components of a compound can’t be separated by physical methods but can be separated by chemical methods only. 5. The properties of a compound are different from that of its elements. 6. The formation of a compound from its elements is accompanied by energy changes. |
1. A mixture is obtained from its (elements, compounds) components as a result of physical change.
2. The mixtures can be homogeneous or heterogeneous. 3. In a mixture the components can be present in any ratio. 4. The components of a mixture can be separated by physical methods only. 5. The properties of a mixture lie between those of its components. 6. The formation of a mixture from its constituents is not accompanied by energy changes. |
Question 6.
How are mixtures classified on the basis of their physical states?
Answer:
On the basis of their physical states, the mixtures are of the following types
| Constituents of mixture | Nature of mixture | Example |
| 1. Solid-Solid mixture | Homogeneous | Brass |
| 2. Solid-Solid mixture | Heterogeneous | Common salt and sand |
| 3. Solid-Liquid mixture | Homogeneous | Sugar in water |
| 4. Solid-Liquid mixture | Heterogeneous | Sulphur in water |
| 5. Liquid-Liquid mixture | Homogeneous | Water and alcohol |
| 6. Liquid mixture | Heterogeneous | Carbon tetrachloride and water |
| 7. Liquid-gas mixture | Homogeneous | Carbon dioxide dissolved in water |
| 8. Gas-Gas mixture | Heterogeneous | A mixture of Nitrogen and Oxygen |
Question 7.
Explain the following:
(a) Fractional crystallisation
(b) Sublimation
(c) Filtration
Answer:
(a) Fractional Crystallisation. The process of separation of components of a mixture having different solubilities in the same solvent is called fractional crystallisation. This method is used for the separation of components of a mixture which are soluble in the same solvent on heating but have different solubilities.
For example, a mixture of potassium nitrate and sodium chloride can be separated by this method.
This can be explained as follows:
The mixture is dissolved in water. The solid is concentrated to the crystallisation point. On cooling the crystals of less slouble component i.e., sodium chloride appear first. These are separated. On further cooling the crystals of more soluble component i.e., of potassium nitrate will appear. The pure crystals can be obtained by recrystallisation from the same solvent i.e, water.
(b) Sublimation: This method is used for the separation of the components of a mixture in which one component undergoes sublimation whereas other does not. For example, a mixture of ammonium chloride and common salt can be separated by this purpose take the mixture of ammonium chloride and common salt in a china dish. Cover it with an inverted funnel and its open end is to be closed with cotton wool. The walls of china dish are kept booled. On heating, ammonium chloride sublimes and condenses on the cooler parts whereas common salt is left behind. The fine powder of ammonium chloride deposited on the funnel is scrapped with the help of a knife.
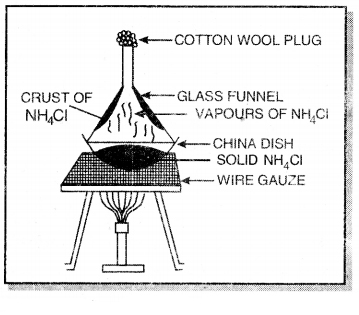
Similarly, the mixture of iodine and sand can be separated by this method in which iodine sublimes whereas sand does not.
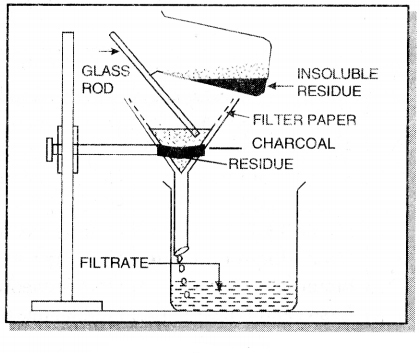
(c) Filtration. In this method, the mixutre is dissolved in a suitable solvent in which one component dissolves whereas other does not.
For example, a mixture of charcoal and sulphur can be separated by this method. The mixture is treated with carbon disulphide which dissloves sulphur whereas charcoal is left behind. The insoluble charcoal is separated by filtration and is dried. From the filtrate, sulphur can be obtained by evaporating carbon disulphide.
Question 8.
Explain the process of:
1. Distillation
2. Evaporation
Answer:
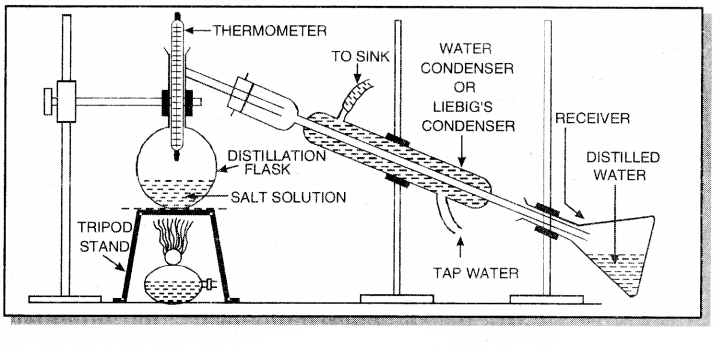
1. Distillation: The process of converting a liquid into gaseous state by heating to boiling point and condensing the vapour to get the pure liquid is called distillation.
For example, salt can be separated from sea water by this method. For this purpose the apparatus is fitted as shown below:
The mixture of solid and liquid is taken in a distillation flask. On heating vapour of liquid (water) are produced. These are condensed in water condenser and collected in a receiver. The non-volatile sodium chloride is left behind. Similar, a mixture of methyl alcohol and iodine i an be separated by this method.
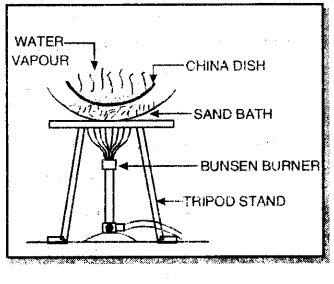
2. Evaporation: Evaporation is a process of changing a liquid into its gaseous state by heating it at a temperature below its boiling point. For example, common salt can be obtained from a mixture of common salt and water by evaporation.
The mixture is heated in a china dish using sand bath when water vaporises whereas common salt is left behind.
Question 9.
What is chromatography? Why is it regarded as superior method of purification?
Answer:
- Chromatography. The process of separation of different components of a mixture by adsorbing them over a suitable material (called adsorbent) is called chromatography.
- Originally, this technique was used to separate coloured mixtures but now-a-days this method can be used for colourless as well as coloured substances.
The main advantages of this technique are:
- It can be applied to separate the mixture even if very small amount of mixture of the substances is available.
- The components of a mixture don’t get wasted during separation.
- It also helps in estimating the constituents of a mixture apart from separation.
Question 10.
What is the principle of chromatographic separation? Name the different types of chromatography commonly used.
Answer:
Chromatography is based upon the distribution of the mixture of the components between the two phases i.e. a stationary phase and a moving phase. The moving phase consists of mixture of the substances to be separated. The moving phase is applied over a solid or liquid i.e., a stationary phase. The stationary phase separates the components of the mixture by the phenomenon of either partition, adsorption or ion exchange. The different chromatographic techniques are:
(a) Column chromatography
(b) Thin layer chromatography
(c) Ion exchange chromatography
(d) Paper chromatography.
Question 11.
How will you separate gas-gas mixtures?
Answer:
The gas-gas mixtures can be separated by the following method:
Fractional evaporation of mixture of liquefied gases. The mixture of two gases is liquefied by applying high pressure and then allowing it to expand. For example, when air is liquefied under high pressure and allowed to stand, both oxygen and nitrogen get liquefied.
The above liquid mixture is maintained at a temperature of -196°C (b.pt. of liquid N2), nitrogen boils off. For example, a mixture of carbon dioxide and hydrogen can be separated by passing through porous partitions as showm below.
In this case, H2 being lighter diffuses at a faster rate as compared to CO2.
Question 12.
What are the various types of colloidal solutions based upon the physical states of dispersed phase and dispersion medium? Give one example in each case.
Answer:
These are of eight types:
| Dispersed Phase | Dispersion Medium | Type or name of colloidal solution | Examples |
| 1. Solid | Solid | Solid sol | Some coloured glasses |
| 2. Liquid | Solid | Gel | Cheese, butter |
| 3. Gas | Solid | Solid foam | Sponge, rubber foam |
| 4. Solid | Liquid | Sol | Mud, milk of magnesia |
| 5. Liquid | Liquid | Emulsion | Milk, hair cream |
| 6. Gas | Liquid | Foam | Froth, whipped cream |
| 7. Solid | Gas | Solid aerosol | Smoke |
| 8. Liquid | Gas | Liquid aerosol | Fog, mist |
Question 13.
Define concentration of a solution. How is concentration of a solution expressed?
Answer:
Concentration of a solution. It indicates the amount of solute dissolved per unit mass or volume of solvent or solution.
The concentration of a solution can be expressed as
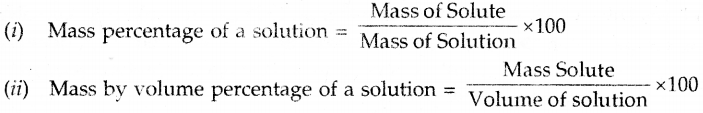
Question 14.
Describe how you would separate the constituents of the following mixtures in a reasonably pure state:
(a) Sand and iodine
(b) Water and sugar
(c) Ingredients of gun powder
(d) Sulphur, sand and common salt
(e) Carbon dioxide and carbon monoxide.
Answer:
(a) Sand and iodine. The mixture can be separated by sublimation when iodine sublimes whereas sand is left behind.
(b) Water and sugar. Sugar and water can be separated by evaporation when water evaporates and sugar is left behind.
(c) Ingredients of gun powder. Gun powder is a mixture of sulphur, nitre and charcoal. Shake the mixture with water. Nitre passes into solution separated by filtration. Evaporate the filtrate. We get pure nitre.
Shake the residue obtained above with carbon disulphide when sulphur dissolves while charcoal is separated by filtration. From filtration sulphur is obtained by evaporating carbon disulphide.
(d) Sulphur, sand and common salt. Shake the mixture with water and filter. Common salt passes into solution. The filtrate is evaporated to get common salt.
Shake the residue obtained above with carbon disulphide and filter sulphur goes into the filtrate. Evaporate it when sulphur is left behind. Sand is left behind insoluble in carbon disulphide.
(e) Carbon dioxide and carbon monoxide. The mixture can be separated by diffusion through a porous pot when carbon monoxide differ faster than carbon dioxide and thus get separated.
Question 15.
What is paper chromatography? How will you separate the coloured constituents present in a mixture of ink and water?
Answer:
The process of separation of different dissolved components of a mixture by adsorbing them on a suitable material (called adsorbent) is called chromatography. The adsorbent can be solid or liquid. For example, alumina, magnesium oxide, special filter paper.
The components of a mixture are generally dissolved in a solvent like water, alcohol etc. If a filter paper is used as an adsorbent for the separation of components of a mixture, this technique is called paper chromatography.
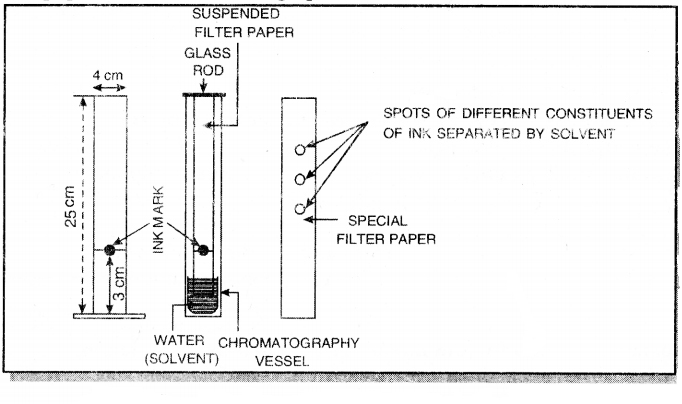
The process of separation of coloured constituents present in a mixture of ink and water is described ahead:
1. Take a special filter paper about 25 cm long and 4 cm broad and stick it to a glass rod at its one end with the help of gum as shown below. Mark a line at a distance of 3 cm from the lower end with the help of pencil. Put a drop of ink at the centre of this line with the help of a fine capillary.
2. Dip this end in water taken in a beaker upto 2 cm.
3. Suspend this filter paper in a tall cylinder and allow it to stand undisturbed for one hour. The water rises up the filter paper and reaches the ink drop, dissolves its components and rise upwards along with water. The different components of ink are adsorbed upto different extents on the filter paper, therefore, they travel different distances on the filter paper.
4. After one hour, the filter paper is taken out and dried. Different of colours corresponding to the components of ink are produced on the filter paper. This filter paper is called chromatograph.
Question 16.
Briefly describe simple methods of separating the following mixtures:
(a) Powdered chalk and sugar
(b) Nitre and common salt
(c) Iron and copper filings
(d) Ammonium chloride, sand and common salt
(e) Ammonia and hydrogen.
Answer:
(a) Powdered chalk and sugar can be separated by using water which dissolves sugar and chalk is insoluble. It is separated by filtration. From sugar solution, sugar is obtained by evaporating water.
(b) Nitre and common salt. The mixture can be separated by fractional crystallisation from the solutions in water because they have different solubilities in water.
(c) Iron and copper filings can be separated by using a magnet when iron filings cling to magnet whereas copper filings don’t.
(d) Ammonium chloride, sand and common salt. Ammonium chloride is separated by sublimation. Sand and common salt are separated by using water when sand remains insoluble and is separated by filtration, From aqueous solution, sodium chloride can be separated by evaporation.
(e) Ammonia and hydrogen can be separated by diffusion through porous pot. Ammonia and hydrogen have different rates of diffusion due to different densities.
Question 17.
How will you separate liquid-liquid mixture or immiscible liquids?
Answer:
The liquid-liquid mixtures can be separated by using :
1. Separating funnel.
2. Fractional distillation.
1. Separation of liquid-liquid mixture by using separating funnel. This method is used when the two liquids are immiscible. For example, a mixture of carbon disulphide and wrater can be separated by this method.
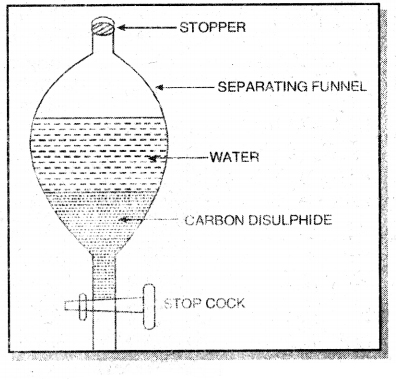
The mixture is taken in a separating funnel. The funnel is allowed to stand for sometime. On standing, the liquid having higher density forms the lower layer whereas the liquid having lower density forms the upper layer. The two liquids are taken out from the separating funnel in separate conical flask.
2. Separation of liquid-liquid mixture using fractional distillation:
The process of separating the mixture of two immiscible liquids by using distillation carried out with the help of a long fractionating column is called fractional distillation.
For example, a mixture of ethyl alcohol and water can be separated by this method. The apparatus is fitted as shown below:
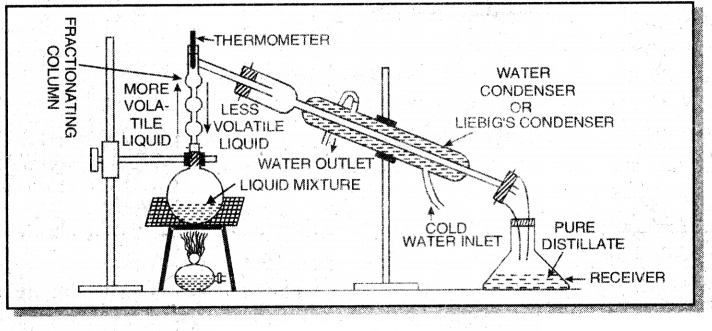
The mixture of ethyl alcohol anci water is heated in a distillation flask fitted with a fractionating column. On heating the vapour of liquid having lower boiling point are produced first and condensed in the water condenser and collected in a receiver. When the temperature of thermometer begins to rise, the vapour of other liquid are produced and these are condensed and collected in a separate receiver.
Question 18.
How will you separate the solid-liquid mixtures?
Answer:
The solid-liquid mixtures can be separated by the following methods:
1. By sedimentation and decantation. In this method, the mixture is allowed to stand when the solid particles settle down as sediment whereas clear liquid is left behind which is poured out carefully. This process is called decantation e.g. mixture of sand and water can be separated by this method. By this method, the complete separation is not possible.
2. By filtration. In this method, the mixture can be separated by using filter paper when the insoluble solid is left on the filter paper whereas the clear liquid passes out from the filter paper and is collected. For example, a mixture of chalk and water can be separated by this method.
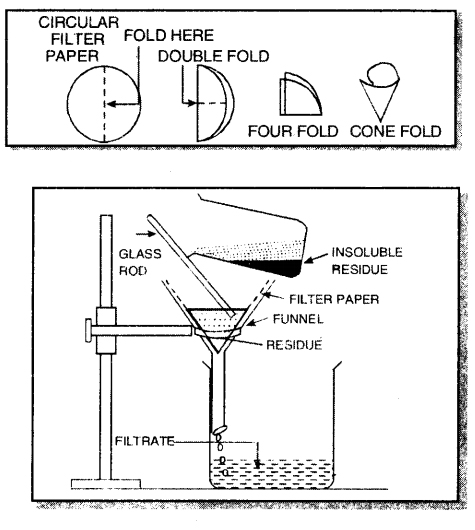
Short Answer Type Questions:
Question 1.
Give three characteristics of a pure substance.
Answer:
- It is homogeneous.
- It has a definite set of properties.
- A pure substance cannot be separated into other kinds of matter by any known physical method.
Question 2.
Give three examples each of two classes of pure substances.
Answer:
Pure substances are of two types:
(a) Elements e.g. carbon, nitrogen, oxygen etc.
(b) Compounds e.g. ammonium, water, carbon dioxide etc.
Question 3.
Give three characteristics of a mixture.
Answer:
(a) The components of a mixture are present in any ratio.
(b) It can be homogeneous or heterogeneous.
(c) The mixture can be separated with its constituents by physical or mechanical methods only.
Question 4.
Name the methods commonly used for separation of components of a mixture.
Answer:
These are:
- Evaporation
- Crytallisation
- Centrifugation
- Using separating funnel
- Simple distillation
- Fractional distillation
- Chromatography
- Sublimation
Question 5.
Define colloidal solution.
Answer:
It is a heterogeneous mixture or solution in which the particles having size 1 to 100 11m are suspended in a suitable solvent or dispersion medium. For example, starch solution.
Question 6.
Define dispersed phase and dispersion medium.
Answer:
- Dispersed phase: In the colloidal solution colloidal particles constitute dispersed phase.
- Dispersion medium: In the colloidal solution the medium in which colloidal particles are dispersed in called dispersion medium.
- e.g. in the colloidal solution of starch in water, starch particles constitutes dispersed phase and water constitutes dispersion medium.
Question 7.
From the methods (or techniques) of distillation, filtration, fractional distillation, chromatography, crystallisation, select and write down the technique you would use to separate:
- Constituents of colouring matter in ink.
- Hydrated copper (II) sulphate or blue vitriol from its aqueous solution.
- Nitrogen from liquid air.
- Unused zinc after the reaction of excess of zinc with dilute sulphuric acid.
Answer:
- Chromatography
- Crystallisation
- Fractional distillation
- Filtration.
Question 8.
Name the technique which could be used to separate:
1. Iodine crystals from sand
2. Petrol from crude oil.
Answer:
1. Iodine crystals can be separated from sand by sublimation.
2. Petrol can be separated from crude oil by fractional distillation.
Question 9.
How will you separate:
1. Pure water from sea water?
2. Kerosene oil from a mixture of kerosene oil and petrol?
Answer:
1. It can be obtained by the process of distillation. When sea water is distilled, water distils, and vapour are condensed in a receiver whereas common salt is left behind in the retort.
2. Kerosene oil can be separated from a mixture of kerosene oil and petrol by fractional distillation when petrol distils off first and then kerosene oil distils off. .
Question 10.
- Name the kind of change of state when naphthalene changes into gaseous state.
- Name one element which undergoes similar change as in (i).
- Name a common substance which exists in all the three states of matter.
Answer:
- Sublimation
- Ammonium chloride
- Water.
Question 11.
Define electrophoresis.
Answer:
Electrophoresis is the process of migration of colloidal particles towards oppositely charged electrode under the influence of an electric field is called electrophoresis. The electrophoresis is due to the charge present on the colloidal particles.
Question 12.
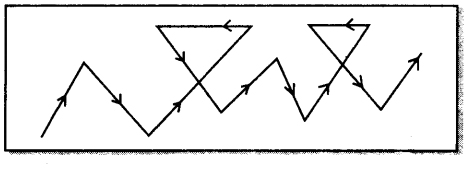
Explain Brownian movement.
Answer:
Brownian movement. When colloidal solutions are viewed with the help of ultra-microscope, it is observed that colloidal particles follow zig-zig path. This is called brownian movement. This effect is due to the unequal impacts of the particles of dispersion medium with colloidal particles.
Question 13.
Explain Tyndall effect.
Answer:
Tyndall effect. If a bright, narrow and convergent beam of light is passed through a colloidal solution and is viewed at right angles with the help of a microscope, the path of light becomes visible and a bright cone of light called Tyndall cone is produced. This luminosity of path of a beam of light in a colloidal solution is called Tyndall effect.
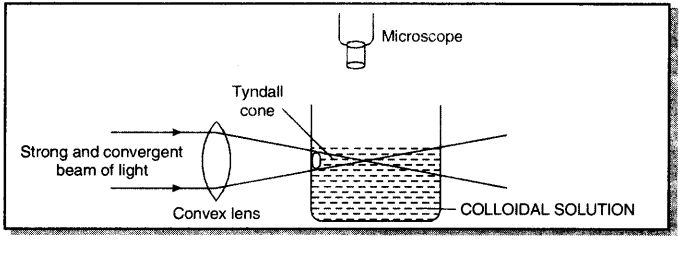
Question 14.
Define physical change and chemical change.
Answer:
- Physical Change. It is a temporary change in which only the physical properties of substances change and it can be easily reversed.
- Examples: Glowing of an electric bulb, evaporation of water.
- Chemical Change: It is a permanent change in which the chemical properties of substances change and there is a change in composition and cannot be reversed.
- Examples: Baking of a cake, Drying of a paint etc.
Question 15.
Classify the following substances into elements, compounds and mixtures:
- Cane sugar
- Zinc
- Oxygen
- Marble
- Bronze
- Nitre
- Air
- Milk
- Iron
- Stainless steel
Answer:
- Cane sugar – Compound
- Zinc – Element
- Oxygen – Element
- Marble – Mixture
- Bronze – Mixture
- Nitre – Compound
- Air – Mixture
- Milk – Mixture
- Iron – Element
- Stainless steel – Mixture
Question 16.
1. Name the process used to separate the constituents liquefied air.
2. State how will you remove carbon dioxide from a mixture of carbon dioxide and carbon monoxide.
Answer:
1. Fractional evaporation.
2. Pass the mixture through potassium hydroxide solution. Carbon dioxide is absorbed by KOH solution.
2KOH + C02 → K2C03 + H20
Carbon monoxide is left behind and is collected over water by downward displacement of water.
Question 17.
How would you separate:
(a) Benzene (b.pt. 80°C) from toluene, methylbenzene (b.pt. 111°C) with which it is miscible?
(b) Kerosene oil from a mixture of kerosene oil and petrol?
(c) Lead sulphate from a mixture of lead sulphate and lead chloride?
Answer:
(a) By using simple distillation when benzene distils off first and its vapours are condensed and collected in a receiver.
(b) Kerosene oil from a mixture of kerosene oil and petrol can be obtained by using separating funnel when kerosene oil forms upper layer.
(c) Lead sulphate from a mixture of lead sulphate and lead chloride can be obtained by boiling with water which dissolves lead chloride and insoluble lead sulphate is separated by filtration.
Question 18.
A molecule can be formed by an element as well as by a compound. Explain.
Answer:
A molecule is the smallest particle of a substance i.e. an element or a compound which can exist freely. It has all the properties of that substance, e.g. a molecule of hydrogen (H2), a molecule of methane (CH4).
Question 19.
Can a mixture of chloroform (b.pt. 61°C) and carbon tetrachloride (b.pt. 77°C) be satisfactorily separated by the process you use for separating the various fractions of petroleum?
Answer:
Yes, a mixture of chloroform and carbon tetrachloride can be separated by using fractional distillation as used for the separation of various fractions of petroleum. The heating should be carried out slowly on a sand bath and vapours are condensed in a Liebig condenser and collected in separate receivers.
Question 20.
Name a mixture used:
- by all living beings
- in the construction of buildings
- as a food
Answer:
- Air
- Cement
- Milk
Question 21.
Give two advantages of paper chromatography.
Answer:
1. It can be applied even to a very small amount of the mixture of the substances.
2. The components of a mixture don’t get wasted during separation.
Question 22.
How would you obtain a sample of sulphur from a mixture of sulphur and carbon?
Answer:
Shake the mixture of sulphur and carbon with carbon disulphide and filter. Sulphur will be present in the filtrate and the residue is carbon. The filtrate is evaporated when carbon disulphide vaporises while sulphur is left behind.
Question 23.
How would you obtain pure ammonium chloride when it contains potassium chloride as an impurity?
Answer:
The mixture is heated. Ammonium chloride sublimes and its vapour are condensed in an inverted funnel. Potassium chloride is left behind. From the funnel ammonium chloride is scrapped with a knife.
Question 24.
Name three metals which are liquids at room temperature.
Answer:
These are mercury, cesium and gallium.
Question 25.
Name the various noble gases.
Answer:
The various noble gases are: Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) and Radon (Rn).
Question 26.
Which of the following statements are correct and which are incorrect?
- Milk is a mixture.
- Smoke is a mixture of solids and gases.
- Ice cream is a mixture.
- Mercury is a solid
- Thums up is a homogeneous mixture.
Answer:
- True
- True
- True
- False
- True.
Question 27.
If you are given a mixture of hydrogen and carbon dioxide, how would you remove the carbon dioxide gas?
Answer:
The carbon dioxide gas can be removed by passing the gaseous mixture through potassium hydroxide solution which absorbs carbon dioxide and hydrogen gas is set free.
Question 28.
Give two pieces of evidence that sodium chloride is a compound.
Answer:
Sodium chloride is a compound because:
1. in sodium chloride, sodium and chlorine are combined chemically in a fixed ratio by mass.
2. the formation of sodium chloride from sodium and chlorine is accompanied by loss of energy.
Question 29.
Give reasons to show that ammonia is a compound.
Answer:
- The properties of ammonia are different from those of its components i.e. nitrogen and hydrogen.
- When ammonia is formed from nitrogen and hydrogen, energy is given out.
- In ammonia, N and H are present in fixed ratio of 14 : 3 by mass.
Question 30.
Give three reasons why air is considered a mixture and not a compound.
Answer:
In air:
- the components are not present in any fixed ratio.
- the properties of air are average of the properties of its components.
- the components can be separated by physical methods.
Question 31.
Define crystallisation. Give its importance.
Answer:
It is a process in which a pure solid in the form of crystals is separated by cooling its hot saturated solution in a suitable solvent.
Importance:
1. For the purification of salt obtained from sea water.
2. To get crystals of alum from impure samples.
Question 32.
1. Some reduced iron filings and powdered roll sulphur are well mixed and heated in a test tube. Describe all what you observe.
2. Name the grey mass which is formed in test tube at the end of reaction.
3. Would you call the above reaction: exothermic or endothermic?
Answer:
1. When iron filings and powdered roll sulphur are well mixed and heated in a test tube, iron (II) sulphide is produced
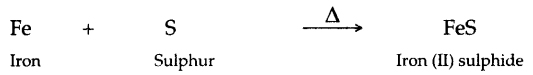
2. Grey mass of iron (II) sulphide is produced.
3. The above reaction is endothermic, because heat energy is absorbed.
Question 33.
What are exothermic and endothermic reactions?
Answer:
1. Exothermic reaction: It is a chemical reaction in which heat energy is evolved. e.g. burning of candle.
2. Endothermic reaction: It is a chemical reaction in which heat energy is absorbed e.g. decomposition of calcium carbonate to give calcium oxide and carbon dioxide.
Question 34.
What is the importance of evaporation?
Answer:
It is used to separate the non-volatile component from volatile solvent component from a mixture e.g. dye can be obtained from blue-black ink.
Question 35.
What are the applications of centrifugation?
Answer:
- It is used in diagnostic laboratories to test blood and urine.
- It is used in diaries and homes to separate butter from cream.
- It is used in washing machines to squeeze out water from wet clothes.
Question 36.
How will you separate a mixture of:
(a) Iodine and sand?
(b) Ammonium chloride and common salt?
(c) Chloroform and water?
(d) Carbon disulphide and water?
Answer:
(a) By sublimation
(b) By sublimation
(c) By using a separating funnel
(d) By using a separating funnel.
Question 37.
A solution contains 40 g of NaCl in 320 g of water. Calculate its concentration in terms of mass percentage of solution.
Answer:
- Mass of NaCl = 40g
- Mass of water = 320g
- Total Mass of solution = 40 + 320 = 360g
- Mass percentage of solution = 40 / 360 × 100 = 100 / 9 = 11.1
Question 38.
What are the advantages of crystallisation over evaporation?
Answer:
- Some solids decompose on heating to dryness.
- Some solids like sugar may get charred on heating.
- Some impurities may be present in the solution on dissolving the impure solid in a solvent.
Question 39.
Name the separation technique to separate:
- Insoluble solid insolvent.
- Solution of solid in liquid.
- Miscible mixture of liquids.
- Immiscible mixture of liquids.
- Mixture of two solids, one of which sublimes
Answer:
- Filtration, centrifugation
- Evaporation, crystallisation, distillation
- Distillation, fractional distillation
- Separating funnel
- Sublimation
Question 40.
What is fractional distillation? Give its importance.
Answer:
Fractional distillation. It is the process of separation of a mixture of two or more miscible liquids having very close boiling points (differing by less than 25°C). In this case, distillation is carried out using a fractionating column.
Importance:
1. This process is ised in the separation of petroleum products.
2. It is used to get component gases from air.
Question 41.
A solution of acetone in water contains 5 ml is 50 ml of its aqueous solution. Calculate the volume percentage of the solution.
Answer:
- Volume of solute = 8ml
- Volume of solution = 50ml
- Volume percentage of solution 5/50 × 100 = 10
Question 42.
Define distillation and give its importance.
Answer:
The conversion of a liquid into vapour and condensing the vapours back into liquid is known as distillation.
Importance:
1. To separate two miscible liquids which boil without decomposition and have sufficient differences in their boiling points (more than 30°C).
2. To separate a volatile component of a solution from a non-volatile component.
Question 43.
How are elements classified?
Answer:
The elements can be classified as:
(a) Metals e.g. Cu, Ag etc.
(b) Non-metals e.g. C, S etc.
(c) Metalloids e.g. As, Sb etc.
(d) Noble gases e.g. He, Ne etc.
Question 44.
Which of the following are pure substances and which are mixtures?
- Air
- Milk
- Graphite
- Gasoline
- Distilled water
- Ice
- Iodised table salt
- Diamond
- Oxygen
- One rupee coin
- 22 Carat gold
- 24 Carat gold
- Steel
- Iron
- Solid Iodine
Answer:
- Air – Mixture
- Milk – Mixture
- Graphite – Pure substance
- Gasoline – Mixture
- Distilled water – Pure substance
- Ice – Pure substance
- Iodised table salt – Mixture
- Diamond – Pure substance
- Oxygen – Pure substance
- One rupee coin – Mixture
- 22 carat gold – Mixture
- 24 carat gold – Pure substance
- Steel – Mixture
- Iron – Pure substance
- Solid Iodine – Pure substance
Question 45.
Which one of the substances given ahead is an element, a mixture or a chemical compound. Give one reason in each case:
(a) chlorine
(b) milk
(c) honey
(d) flowers of sulphur
(e) seawater
(f) gun powder
(g) brine
(h) sulphur
(i) apple juice
(j) carbon dioxide
(k) air
(Z) syrup.
Answer:
(a) Chlorine. It is an element made up of only one kind of atom.
(b Milk. It is a mixture of fat, water etc.
(c )Honey. It is a mixture of carbohydrates.
(d Flowers of sulphur. It is an element made up of only sulphur molecules (S8).
(e )Sea Water. It is a mixture of water and dissolved salts.
(f) Gun powder. It is a mixture of sulphur, nitre and charcoal.
(g) Brine. It is a mixture of water and sodium chloride.
(h) Sulphur. It is an element made up of sulphur molecules (S8).
(i) Apple juice. It is a mixture.
(j) Carbon dioxide. It is a compound of carbon and oxygen combined together in the fixed ratio 3 : 8 by mass.
(k) Air. It is a mixture of oxygen, nitrogen and other gases.
(Z) Syrup. It is a mixture.
Question 46.
(a) Name a chemical technique which could be used successfully to separate
1. Iodine crystals from sand.
2. Petrol from kerosene oil.
(b) A pupil decides to separate powdered calcium carbonate from powdered calcium chloride by shaking the mixture with water and filtering. Would this procedure succeed? Explain your answer.
(c) Mixtures are usually heterogeneous, but sometimes homogeneous also.
Give one example each of two different types of mixtures.
Answer:
(a) 1. Sublimation
2. Fractional distillation
(b) Yes, when a mixture of calcium carbonate and calcium chloride is treated with water, calcium chloride dissolves whereas calcium carbonate does not. It is separated by filtration.
(c) Homogeneous mixture, e.g. Brass is a homogeneous mixture of copper and zinc.
Heterogeneous mixture, e.g. common salt and sand.
Question 47.
From the following techniques:
Distillation, filtration, fractional distillation, chromatography, crystallisation, sublimation, evaporation, decantation, sedimentation. Select the method you will use to separate:
(a) the constituents of the colouring matter of ink.
(b) hydrated CuS04 from its aqueous solution.
(c) Grass stains.
(d) Common salt from sea-water.
(e) Petrol from crude oil.
Answer:
(a) Chromatography
(b) Crystallisation
(c) Chromatography
(d) Evaporation
(e) Fractional distillation.
Question 48.
How will you separate a mixtu funnel? Explain with an activity.
Answer:
Take a clean separating funnel and take a mixture of mustard oil and water in it. Allow it to stand for sometime. On standing, two separate layers appears. The lower layer is of water (heavier) and the upper layer is of mustard oil (lighter).
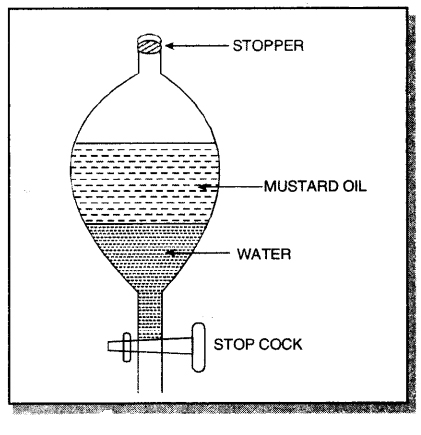
Open the stop cock of the separating funnel and pour out the lower layer of water carefully. Close the stop cock when the water has been removed. Mustard oil is left in the separating funnel.
Very Short Answer Type Questions:
Question 1.
Define pure substance.
Answer:
It is a material containing particles of only one kind having a definite set of properties.
Question 2.
Define mixture.
Answer:
It is a material obtained by mixing two or more elements and or compounds in any ratio.
Question 3.
How will you separate the components of an aqueous solution of sodium chloride?
Answer:
By simple distillation.
Question 4.
Give two examples of homogeneous mixture.
Answer:
Sodium chloride dissolved in water, sugar dissolved in water.
Question 5.
Give two examples of homogeneous mixtures.
Answer:
Iron filings and common salt, common salt and sulphur.
Question 6.
Is the mixture of oil and water, homogeneous or heterogeneous?
Answer:
Heterogeneous.
Question 7.
What is solution?
Answer:
It is a homogeneous mixture of two or more substances whose composition can be changed within certain fixed limits.
Question 8.
Give examples of solid solution.
Answer:
Brass, Bronze.
Question 9.
Give one example of gaseous solution.
Answer:
Air.
Question 10.
Name the components of Brass.
Answer:
Zinc and copper.
Question 11.
Define solute.
Answer:
It is the component of a solution which is present in small amount and which is dissolved in another component called solvent.
Question 12.
Define solvent.
Answer:
It is the component of solution which is present in large amount and in which solute is dissolved.
Question 13.
Name the solute and solvent in an aqueous solution of sugar.
Answer:
Water-Solvent, Sugar-Solute.
Question 14.
In tincture of Iodine, name solute and solvent.
Answer:
Solute-Iodine, Solvent-Alcohol.
Question 15.
In soda water, name solute and solvent.
Answer:
Solute-Carbon dioxide, Solvent-water.
Question 16.
What is the type of air mixture?
Answer:
Homogeneous.
Question 17.
In air name solute and solvent?
Answer:
Solvent-Nitrogen, Solute-Oxygen and other gases.
Question 18.
What is the size of particles of solutions?
Answer:
Less than lnm (10-9m).
Question 19.
What is saturated solution?
Answer:
It is a solution in which no more of solute can be dissolved at the given temperature and pressure.
Question 20.
What is unsaturated solution?
Answer:
It is a solution in which more of solute can be dissolved at the given temperature and pressure.
Question 21.
What is the concentration of a solution?
Answer:
It gives us the exact amount of solute dissolved in an exact amount of solvent or solution.
Question 22.
What is a suspension?
Answer:
It is a heterogeneous mixture in which small particles of a solid don’t dissolve but remain suspended in the liquid (or gas).
Question 23.
Why is suspension temporary?
Answer:
This is because components of suspension can be saparated by filtration.
Question 24.
Suspended particles in a suspension can be seen with naked eye, why?
Answer:
This is because their sizes are more than 100 run.
Question 25.
What is Tyndall effect?
Answer:
It is the scattering of light by colloidal particles.
Question 26.
How will you prove that milk is a colloidal solution?
Answer:
It shows Tyndall effect.
Question 27.
What is the size of colloidal particles?
Answer:
1 to 100 nm.
Question 28.
How will you separate colloidal particles from solution?
Answer:
By ultra centrifugation.
Question 29.
When do we don’t use filtration?
Answer:
If the solid particles present in solution have very small sizes and can pass through pores of filter paper.
Question 30.
What is the principle of centrifugation of mixtures?
Answer:
Heavier particles settle down and lighter particles float over them.
Question 31.
Where do we use centrifugal machine?
Answer:
In diagnostic laboratory to study urine and blood, in dairies to separate butter and cream from milk, to squeeze water from clothes in washing machine.
Question 32.
On the basis of what principle, two immiscible liquids can be separated?
Answer:
Based upon density.
Question 33.
How can a mixture of sodium chloride and ammonium chloride separated?
Answer:
By sublimation.
Question 34.
Name four substances which undergo sublimations.
Answer:
Ammonium chloride, comphor, naphthalene, anthracene.
Question 35.
How many colours are mixed in a dye?
Answer:
Two or more colours.
Question 36.
Define chromatography.
Ans.wer:
It is the separation of coloured components of a mixture based upon adsorption.
Question 37.
What are the uses of chromatography?
Answer:
1. To separate components of a dye.
2. To separate colours from natural colours, to separate sugar from urine and to separate medicine from blood.
Question 38.
Which type of liquid mixture solutions can be separated by simple distillation?
Answer:
Liquids having large differences in their boiling points.
Question 39.
Which type of liquid mixture solutions are separated by fractional distillation?
Answer:
Liquids whose boiling points differ by less than 25 K.
Question 40.
Give two examples where fractional distillation is used to separate the components of a mixture.
Answer:
Air, petroleum.
Question 41.
What is the function of glass beads in fractionating column?
Answer:
These provide surface for cooling and condensing vapours.
Question 42.
Which type of mixture air is?
Answer:
It is a homogeneous mixture.
Question 43.
What is the boiling point of oxygen?
Answer:
183°C.
Question 44.
What is the boiling point of Argon.
Answer:
186°C.
Question 45.
What is the boiling point of nitrogen?
Answer:
196°C.
Question 46.
When do we use crystallisation for purification?
Answer:
To purify solids.
Question 47.
How will you get pure copper sulphate?
Answer:
By crystallisation.
Question 48.
Why is crystallisation better than simple distillation?
Answer:
In simple distillation, some substances decompose or are burnt and some impurities are left.
Question 49.
How will you get pure water from sea water?
Answer:
By evaporation and simple crystallisation.
Question 50.
How is alum purified?
Answer:
By crystallisation.
Question 51.
What are the changes during chemical change?
Answer:
Chemical decomposition and change of chemical properties.
Question 52.
Name the type of change of combustion?
Answer:
Chemical change.
Question 53.
How is matter classified on the basis of chemical decomposition?
Answer:
Elements and compounds.
Question 54.
Which French scientist proved that element is a fundamental or basic particle of matter?
Answer:
Antonie Lorentz Lavosier.
Question 55.
Which is the basic unit of matter?
Answer:
Element.
Question 56.
Define an element.
Answer:
It is the smallest particle of an element which can’t be decomposed into still smaller particles by chemical reaction.
Question 57.
How are elements classified?
Answer:
Metals, non-metals and metalloids.
Question 58.
Name the metal which is liquid at room temperature.
Answer:
Mercury.
Question 59.
What is metalloid?
Answer:
It shows the properties of both metals and non-metals.
Question 60.
Give two examples of metalloids.
Answer:
Arsenic, antimony.
Question 61.
Give examples of non-metals.
Answer:
Carbon, hydrogen, oxygen, chlorine, bromine etc.
Question 62.
Give examples for metals.
Answer:
Sodium, potassium, copper, iron, gold, silver etc.
Question 63.
How many elements are known till today?
Answer:
112.
Question 64.
How many elements occur in nature?
Answer:
There are 92 natural elements.
Question 65.
How many elements are gases at room temperature?
Answer:
11.
Question 66.
Name five methods to separate mixtures.
Answer:
- Filtration
- Crystallisation
- Sublimation
- Evaporation
- Distillation.
Question 67.
How are sugar crystals separated in a sugar factory?
Answer:
By centrifugation process.
Question 68.
The size of colloidal particals is in the range …………….. .
Answer:
1 to 100 run.
Question 69.
Tyndall effect is shown by ……………. .
Answer:
Colloidal particles.
Question 70.
He and Ne are ……………….. gases.
Answer:
Noble.
Question 71.
FeS + H2S04 → …………..
Answer:
FeS + H2S04 → FeS04 + H2S
Question 72.
In a refinery, petrol is obtained from crude oil by the process of …………… .
Answer:
Fractional distillation
Question 73.
Grass stains are removed from the clothing by using ……………. as solvent.
Answer:
Methylated spirit
Question 74.
Common salt is obtained from sea water by the process of ……………. .
Answer:
Evaporation
Question 75.
When caustic soda solution is added to an aqueous solution of copper sulphate, a blue precipitate of copper hydroxide is obtained. The copper hydroxide can be separated from the mixture by the process of ……………….. .
Answer:
Filtration.
Science Guide for Class 9 PSEB Is Matter Around Us Pure InText Questions and Answers
Question 1.
What is meant by a pure substance?
Answer:
It is a material containing particles of only one kind having a definite set of properties. Pure substances include elements and compounds.
Question 2.
List the points of differences between homogeneous and heterogeneous mixtures.
Answer:
| Homogeneous mixture | Heterogeneous mixture |
| 1. It consists a single phase.
2. It has a uniform composition throughout. 3. It has the same properties throughout the bulk. 4. There are no visible boundaries between its components. 5. Examples: |
1. It consists two or more phases.
2. It does not have a uniform composition thoughout. 3. It does not have same properties throughout the bulk. 4. There are visible boundaries of separation between its components. 5. Examples: |
Question 3.
Differentiate between homogeneous and heterogeneous mixtures with examples.
Answer:
Same as given above.
Question 4.
How are true solution, colloidal solution and suspension different from each other?
Answer:
| Property | True Solution | Colloidal solution | Suspension |
| 1. Nature | Homogeneous | Heterogeneous | Heterogeneous |
| 2. Particle size | Less than 1 am | 1 to 100 nm | More than 100 nm |
| 3. Diffusion | Diffuse rapidly | Diffuse slowly | Don’t diffuse |
| 4. Filtrability | Paritcles can pass through ordinary filter paper as well as semipermeable membrane | Particles can pass through ordinary filter paper but not through semi-permeable membrane | Particles can’t pass through filter paper as well as semipermeable membrane |
| 5. Appearance | Clear and transparent | Generally clear and transparent | Opaque |
| 6. Tyndall effect | Don’t show | Show | May or may not show |
| 7. Visibility | Particles are not visible | Particles can be seen only with ultramicroscope | Particles can be seen with naked eye or microscope. |
Question 5.
To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
Answer:
Mass of sodium chloride (solution) = 36g
Mass of water (solvent) = 100g
Total mass of solution = 100 + 36 = 136g
Mass percentage of solution = Mass of solute / Mass of solution × 100
= 36 / 136 × 100
= 26.47
Question 6.
How will you separate a mixture containing kerosene and petrol (difference in their boiling points is more than 25°C), which are miscible with each other?
Answer:
By distillation.
Question 7.
Name the technique to separate:
- Butter from curd
- Salt from seawater
- Comphor from salt.
Answer:
- By centrifugation.
- By evaporation.
- By sublimation.
Question 8.
What types of mixture are separated by the technique of crystallisation?
Answer:
The mixtures in which the substance is soluble in a suitable hot solvent whereas the impurities are insoluble.
Question 9.
Classify the following as chemical or physical changes:
(a) cutting of trees.
(b) melting of butter in a pan.
(c) rusting of almirah.
(d) boiling of water to form steam.
(e) passing of electric current through water and the water breaking down into hydrogen and oxygen gases.
(f) dissolving common salt in water.
(g) making a fruit salad with raw fruits.
(h) burning of paper and wood.
Answer:
(a) Cutting of trees-Chemical change
(b) Melting of butter in a pan-Physical change
(c) Rusting of almirah-Chemical change
(d) Boiling of water to form steam-Physical change
(e) Passing of electric current through water and the water breaking down into hydrogen and oxygen gases-Chemical change
(f) Dissolving common salt in water-Physical change
(g) Making a fruit salad with raw fruits-Chemical change
(h) Burning of paper and wTood- Chemical change
Question 10.
Try segregating the things around you as pure substances or mixtures.
Answer:
Some examples are:
- Water – Pure substance
- Milk – Mixture
- LPG – Mixture
- Bread – Mixture
- Kerosene oil – Mixture
- Air – Mixture
- Paper – Mixture
- Ink – Mixture
- Curd – Mixture
- Wood – Mixture
- Ice – Pure substance
- Soda Water – Mixture
- Ice cream – Mixture
- Butter – Mixture
- Vanaspati ghee – Mixture
- Lemon juice – Mixture
PSEB 9th Class Science Guide Is Matter Around Us Pure Textbook Questions and Answers
Question 1.
Which separation techniques will you apply for the separation of the following?
(a) Sodium chloride from its solution in water.
(b) Ammonium chloride from a mixture containing sodium chloride and ammonium chloride.
(c) Small pieces of metal in the engine oil of a car.
(d) Different pigments from an extract of flower petals.
(e) Butter from curd
(f) Oil from water
(g) Tea leaves from tea
(h) Iron pins from sand
(i) Wheat grains from husk
(j) Fine mud particles suspended in water.
Answer:
(a) Evaporation
(b) Sublimation
(c) Filtration
(d) Chromatography
(e) Centrifugation
(f) Separating funnel
(g) Filtration
(h) Magnetic separation
(i) Gravity separation
(j) Centrifugation
Question 2.
Write the steps you would use for making tea. Use the words0solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue.
Answer:
Step 1: Boil some solvent (water ) in a pan.
Step 2: Put some solute (tea leaves) in a tea pot.
Step 3: Pour the boiling water into the tea pot and let it soak for a few minutes to form a solution.
Step 4: Stir the solution in the tea pot.
Step 5: Take some solute (sugar) into a cup.
Step 6: Filter the above solution obtained in step 4 using strainer and take this solution (filtrate) in the cup. Pour two tea-spoons of milk into the cup and stir it with a spoon. This gives us tea which is ready for drinking. The residue is left on the strainer because these are insoluble whereas sugar and milk are soluble solutes.
Question 3.
Pragya tested the solubility of three different substances at different temperatures and collected the data as given below (results are given in the following table, as grams of substance dissolved in 100 grams of water to form a saturated solution).
| Substances Dissolved | Temperature in K | ||||
| 283 | 293 | 313 | 333 | 353 | |
| Potassium nitrate | 21 | 32 | 62 | 106 | 167 |
| Sodium chloride | 36 | 36 | 36 | 37 | 37 |
| Potassium chloride | 35 | 35 | 40 | 46 | 54 |
| Ammonium chloride | 2.4 | 37 | 41 | 55 | 66 |
(a) What mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 grams of water at 313 K?
(b) Pragya makes a saturated solution of potassium chloride in water at 353 K and leaves the solution to cool at room temperature. What would she observe as the solution cools? Explain.
(c) Find the solubility of each salt at 293 K. Which salt has the highest solubility at this temperature?
(d) What is the effect of change of temperature on the solubility of a salt?
Answer:
(a) Solubility of KN03 in water at 313 K = 62 g / 100 g of water
100 g of water contains KN03 at 313 K = 62 g
∴ 50 g of water will contains KN03 at 313 K = 62/100 × 50/1 = 31 g
∴ Amount of potassium nitrate needed to produce a saturated solution of potassium nitrate in 50 g of water at 313 K = 31 g
(b) On cooling the saturated solution of potassium chloride in water at 353 K to room temperature, the solution will become supersaturated at room temperature and some potassium chloride will settle down.
(c) Solubility of potassium nitrate at 293 K = 32g/100g of water
Solubility of sodium chloride at 293 K = 36g/100g of water
Solubility of potassium chloride at 293 K = 35g/100g of water
Solubility of ammonium chloride at 293 K = 37g/100g of water
Ammonium chloride has highest solubility at 293 K.
(d) (1) If the process of dissolution of salt in water is exothermic, its solubility decreases with the increase in temperature.
(2) If the process of dissolution of salt in water is endothermic, its solubility increases with the increase in temperature.
Question 4.
Explain the following giving examples:
(a) saturated solution
(b) pure substance
(c) colloid
(d) suspension.
Answer:
(a) Saturated Solution: A solution in which no more of the solute can be dissolved at the given temperature is said to be saturated at that temperature. For example 50 g of NaCl added to 100 ml of water.
(b) Pure substance: A pure substance is made of only one type of atoms or molecules. Pure substances have the same colour, taste, texture at the given temperature and presure. Pure substance has a fixed melting or boiling point at the constant pressure. Pure substance includes elements and compounds. For example, hydrogen gas, sodium chloride, water etc.
(c) Colloid: A substance is said to be in the colloidal state if its particle size lies between 1 to 100 nm. The colloidal solution is heterogeneous and consist of two phases i.e. dispersed phase or colloidal particles and dispersion medium in which colloidal particles are suspended e.g. colloidal solution of starch in water.
(d) Suspension: It is a heterogeneous mixture in which the particles of the solute don’t dissolve but remain suspended throughout the bulk of the material and the size of particles is more than 10-7 m. The particles can be seen with naked eye.
Question 5.
Classify each of the following as a homogeneous or heterogeneous mixture: soda water, wood, air, soil, vinegar, filtered tea.
Answer:
- Soda Water – Homogeneous mixture
- Wood – Heterogeneous mixture
- Air – Homogeneous mixture
- Soil – Heterogeneous mixture
- Vineger – Homogeneous mixture
- Filtered tea – Homogeneous mixture.
Question 6.
How would you confirm that a colourless liquid given to you is pure water?
Answer:
Determine the boiling point of the given liquid. If its boiling point is 100°C at 1 atmosphere pressure it is pure water and if boiling point is above 100°C, it is impure water.
Question 7.
Which of the following materials fall in the category of a “pure substance”?
(a) Ice
(b) Milk
(c) Iron
(d) Hydrochloric acid
(e) Calcium oxide
(f) Mercury
(g) Brick
(h) Wood
(i) Air
Answer:
(a) Ice
(b) Iron
(c) Hydrochloric acid
(d) Calcium oxide
(f) Mercury
Question 8.
Identify the solutions among the following mixtures:
(a) soil
(b) sea water
(c) air
(d) coal
(e) soda water
Answer:
(b) sea water
(c) air and
(e) soda water
Question 9.
Which of the following will show “Tyndall effect”?
(a) Salt solution
(b) Milk
(c) Copper sulphate solution
(d) Starch solution.
Answer:
(b) Milk and (d) Starch solution because these are colloidal solutions.
Question 10.
Classify the following into elements, compounds and mixtures:
(a) sodium
(b) soil
(c) sugar solution
(d) silver
(e) calcium carbonate
(f) tin
(g) silicon
(h) coal
(i) air
(j) soap
(k) methane
(l) carbon dioxide
(m) blood
Answer:
(a) Sodium – Element
(b) Soil – Mixture
(c) Sugar solution – Mixture
(d) Silver – Elemei
(e) Calcium carbonate – Compound
(f) Tin – Element
(g) Silicon – Element
(h) Coal – Mixture
(i) Air – Mixture
(j) Soap – Mixture
(k) Methane – Compound
(l) Carbon dioxide – Compound
(m) Blood – Mixture
Question 11.
Which of the following are chemical changes?
(a) Growth of a plant
(b) Rusting of iron
(c) Mixing of iron filings and sand
(d) Cooking of food
(e) Digestion of food
(f) Freezing of water
(g) Burning of a candle.
Answer:
(a) Growth of a plant
(b) Rusting of iron
(d) Cooking of food
(e) Digestion of food
(f) Burning of a candle.
Follow on Facebook page – Click Here
Google News join in – Click Here
Read More Asia News – Click Here
Read More Sports News – Click Here
Read More Crypto News – Click Here