WBBSE 10th Class Science Solutions Physical Science & Environment Chapter 7 Atomic Nucleus
WBBSE 10th Class Science Solutions Physical Science & Environment Chapter 7 Atomic Nucleus
West Bengal Board 10th Class Science Solutions Physical Science & Environment Chapter 7 Atomic Nucleus
WBBSE 10th Class Physical Science & Environment Solutions
Synopsis
Radioactivity
- Radioactive elements are those elements whose atoms spontaneously emit a type of special invisible radiation and get converted into atoms of new elements. Radioactivity was discovered by Henri Becquerel in uranium salt in the year 1896.
- Radioactivity is the property due to which some elements with high atomic mass numbers spontaneously emit a type of special invisible radiation and get converted into atoms of new elements.
- All the elements with atomic number greater than 82 are naturally radioactive.
- Some non-radioactive elements may be converted into elements radioactive artificially. The radioactivity of these new elements produced artificially is called artificial radioactivity.
- The phenomenon of spontaneous emission of rays from an unstable nucleus is called radioactive decay.
- Radioactive element or atom that exhibits radioactivity is called parent atom. The atom that is left behind after the emission of radioactive radiation is called daughter atom.
- No radioactive sample can emit all three radiations simultaneously.
- Three types of rays are emitted in radioactivity.
α -ray: It is a stream of positively charged particles.β -ray: It is a stream of negatively charged particles.γ -ray: It is an electromagnetic wave.
- If an atomic nucleus undergoes a -decay, the atom is transformed into an atom with a mass number that is reduced by four units and an atomic number that is reduced by two units.
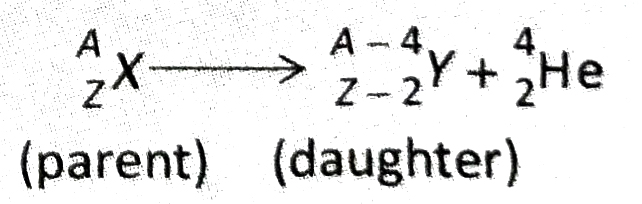
- If an atomic nucleus undergoes B-decay, the atom is transformed into another atom whose mass number remains unchanged and the atomic number increases by one unit.
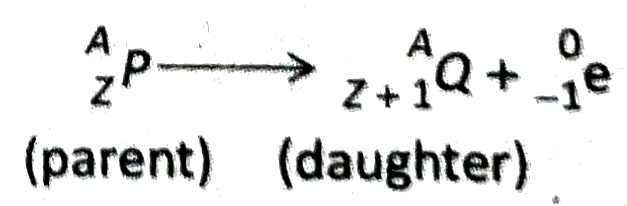
- If γ -ray is emitted from a nucleus, the mass number and atomic number of the atom remain unchanged.
TOPIC – A
Radioactivity
SHORT AND LONG ANSWER TYPE QUESTIONS
Q.1 What is radioactivity? Which element does show radioactivity?
Ans. Radioactivity is the property due to which some elements with high atomic mass numbers spontaneously emit a type of special invisible radiation and get converted into atoms of new elements.
Elements in which the ratio of neutrons and protons in the nuclei is more than 1.5, the nuclei are unstable. These elements emit radioactive radiation spontaneously and try to become stable. Only that element shows radioactivity whose mass number is 210 or more than that.
Q.2 What are the characteristics of radioactivity?
Ans. The characteristics of radioactivity are:
- Radioactive rays emit spontaneously and ceaselessly from a radioactive element.
- No effect does occur in the emission of radioactive rays even in application of heat, pressure, light, electric field or magnetic field on the radioactive substance.
- If radioactive elements undergo chemical change and form other compounds, there is no change in their radioactive nature.
- Radioactivity of an element is a phenomenon fully associated with its nucleus, there is no connection with its electronic configuration.
- Structure of the nucleus changes as a result of emission of radioactive rays. In other words, the numbers of proton and neutron in the nucleus change and as a result, a new element is formed.
Q.3 Write down three characteristics of radioactive radiation.
Ans. Characteristics of radioactive radiation:
- The radiation has a high penetrating power. As for example, gamma rays require 1 cm thick lead slab to reduce their intensity by 50%.
- Radioactive rays can ionise a gas.
- This radiation affects photographic plate.
Q.4 What is artificial radioactivity? Name three artificial radioactive elements.
Ans. Some non-radioactive elements may be converted into radioactive elements artificially. The radioactivity of these new elements produced artificially is called artificial radioactivity.
Three artificial radioactive elements are
Q.5 Radioactivity of any element is completely a nuclear phenomenon-explain.
Ans. The property of radioactivity of radioactive elements remains unchanged even when they form other compounds after their chemical change. For example, radium chloride, a compound of radioactive radium, is also radioactive. The chemical properties of an element depend on the electronic configuration in its different orbits. As the radioactivity of a radioactive element remains present after chemical changes, so there is no relation between radioactivity and electronic configuration of the element. Furthermore, an element with new properties is formed as a result of the emission of radioactive rays. This is possible only if there is a change in the structure of the nucleus, i.e., a change in the number of neutrons and protons in the nucleus. This is why it is said that radioactivity of any element is completely a nuclear phenomenon.
Q. 6 Define parent atom and daughter atom. What is the position of a daughter atom in the periodic table when one a-particle is emitted from the nucleus of a radioactive element? What is the position of a daughter atom in the periodic table when one ßparticle is emitted from the nucleus of a radioactive element?
Ans. Parent atom of the radioactive element is that atom which undergoes radioactive decay. The atom of the new element which is produced after emission of radioactive ray is called the daughter atom.
If one a -particle is emitted from the nucleus of a radioactive element, the atomic number of the daughter atom is reduced by two units. So the daughter element moves 2 places to the left in the periodic table compared to the parent atom. If one B-particle is emitted from the nucleus of a radioactive element, the atomic number of the daughter atom is increased by one unit. So the daughter element moves 1 place to the right in the periodic table compared to the parent atom.
Q.7 Write down the differences between a β-particle and an ordinary electron.
Ans. Differences between a β-particle and an ordinary electron:
| β-particle | Ordinary electron |
| 1. β-particle is emitted instantaneously after its creation in the nucleus of a radioactive atom. | 1. Ordinary electrons remain in different orbits outside the nucleus of the atom. |
| 2. After emission of a β-particle from the nucleus of a radioactive element, the mass number of the new element remains the same but atomic number increases by one unit. | 2. An atom is converted into a positively charged ion after emission of an ordinary electron from the atom. |
Q.8 Write down the differences between a radioactive change and a chemical change.
Ans. The differences between a radioactive change and a chemical change:
| Radioactive change | Chemical change |
| 1. Radioactive change is a nuclear phenomenon. | 1. Chemical change is a phenomenon involving valence electrons in the orbits of the atom. |
| 2. Radioactive change is not influenced by external pressure, light, catalyst, electric field or magnetic field. | 2. Chemical change is influenced by external pressure, light, catalyst etc. |
| 3. Radioactive change is a irreversible change. | 3. Chemical change may be irreversible as well as reversible. |
| 4. A new element is formed in a radioactive change. | 4. No news element is formed in a chemical change. |
| 5. Huge amount of energy is produced in a radioactive change. | 5. Amount of energy produced in a chemical change is less than that produced in a radioactive change. Moreover in some cases, energy is absorbed in a chemical change. |
Q.9 Mention three applications of radioactivity.
Ans. Radioactive elements are used at present in many areas including medical science.
- Radioactive elements radium, cobalt (60Co) are used to destroy those cells which are affected by cancer. Radioactive phosphorus (32P) is used for the treatment of leukemia and brain tumour. Radioactive iodine (131I) is used for the treatment of disease of thyroid.
- Radioactive elements are used to determine the suitability of certain raw materials used in different industrial units.
- A smoke detector has been manufactured on the basis of a special characteristic of the radioactive material (americium-241). This instrument can detect smoke which is emitted due to an accidental fire, and alarm the people by the sound of a siren.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. Among the following rays, which one has the maximum power to ionise gases?
A. α
B. β
C. γ
D. visible ray
Ans. A
2. Among the following rays, which one has the maximum penetrating power?
A. α
B. β
C. γ
D. visible light
Ans. C
3. Among the following isotopes, which one is radioactive?
A. 12C
B. 14C
C. 16O
D. 23Na
Ans. B
4. Speed of γ-ray in vacuum is
A. 2 × 108m/s
B. 3 × 108m/s
C. 1.5 × 108m/s
D. 2.5 × 108m/s
Ans. B
5. Charge of an α -particle is
A. 1.6 × 10-19 C
B. 3.2 × 10-19 C
C. 4.8 × 10-19 C
D. 6.4 × 10-19 C
Ans. B
6. What is the mass of a β-particle, if the mass of an electron is m?
A. m
B. 2m
C. 3m
D. 4m
Ans. A
7. Which of the following is used for determination of the age of fossils?
A. 60CO
B. 131I
C. 32P
D. 14C
Ans. D
8. Who discovered radioactivity?
A. Ernest Rutherford
B. Madame Curie
C. Pierre Curie
D. Henry Becquerel
Ans. D
9. If a powerful static electric field is applied in a perpendicular direction to the path of radioactive rays, which ray does bend towards the positively charged terminal?
A. α
B. β
C. γ
D. none of these
Ans. B
10. If a powerful static electric field is applied in a perpendicular direction to the path of radioactive rays, which ray does bend towards the negatively charged terminal?
A. α
B. β
C. γ
D. none of these
Ans. A
11. If a powerful static electric field is applied in a perpendicular direction to the path of radioactive rays, which ray does not deviate?
A. α
B. β
C. γ
D. none of these
Ans. C
12. Which of the following has a speed equal to that of light?
A. α -ray
B. β -ray
C. γ -ray
D. none of these
Ans. C
13. How many β-particles in succession to one à -particle need to be emitted from the nucleus of an atom of X to make the new element an isotope of X?
A. 1
B. 2
C. 3
D. 4
Ans. B
14. If m is the mass of an electron, then the mass of a positron is
A. m
B. m/2
C. 2m
D. 4m
Ans. A
15. Particles which can be added to the nucleus of an atom without changing its chemical properties are
A. neutrons
B. electrons
C. protons
D. positrons
Ans. A
16. Which of the following particles are constituents of the nucleus?
A. protons and electrons
B. neutrons and electrons
C. electrons and positrons
D. neutrons and protons
Ans. D
17. The mother and daughter elements with the emission of β-rays are
A. isotopes
B. isobars
C. isomers
D. isodiaphers
Ans. B
18. The mother and daughter elements with the emission of γ -rays are
A. isotopes
B. isobars
C. isomers
D. isodiaphers
Ans. C
19. Atomic number of a nucleus is Z and atomic mass is M. The number of neutron is
A. M – Z
B. M
C. Z
D. M + Z
Ans. A
Answer in brief
1. α-ray is composed of which type of electrically charged particles?
Ans. α-ray is composed of positively charged particles.
2. β-ray is composed of which type of electrically charged particles?
Ans. β-ray is composed of negatively charged particles.
3. Among α -ray, β -ray and γ -rays, which one is electrically neutral?
Ans. γ-ray is electrically neutral.
4. Is α -ray scattered by electric field?
Ans. Yes, α -ray is scattered by electric field.
5. What are radioactive elements?
Ans. Radioactive elements are those elements whose atoms spontaneously emit a type of special invisible radiation and get converted into atoms of new elements.
6. Write down the names of three natural radioactive elements.
Ans. Polonium (Po), radium (Ra) and uranium (U) are three natural radioactive elements.
7. Write down the name of an instrument which measures radioactivity.
Ans. G-M Counter (Geiger-Muller Counter) measures radioactivity.
8. Which element is responsible for the radioactivity of potassium uranyl sulphate?
Ans. Uranium is responsible for the radioactivity of potassium uranyl sulphate.
9. What is the effect of radioactivity on the cells of a living organism?
Ans. The effect of radioactive rays on a cell may be fatal as it may destroy active cells.
10. How many protons in the nucleus are reduced if one a -particle is emitted from the nucleus of a radioactive element?
Ans. Two protons are reduced in the nucleus, if one a -particle is emitted from the nucleus of a radioactive element.
11. How many neutrons in the nucleus are reduced if one a-particle is emitted from the nucleus of a radioactive element?
Ans. Two neutrons are reduced in the nucleus, if one a -particle is emitted from the nucleus of a radioactive element.
12. How many types of ray are emitted from a radioactive substance? Write down their names.
Ans. Three types of ray are emitted from a radioactive substance, namely alpha (α) ray, beta (β) ray and gamma (γ) ray.
13. Among the rays which are emitted from a radioactive material, which is an electromagnetic wave?
Ans. Among the rays which are emitted from a radioactive material, gamma ray is an electromagnetic wave.
14. Of all the rays emitted from a radioactive material, which rays have particle nature?
Ans. Of all the rays emitted from a radioactive material, α-ray and β-ray are streams of particles with masses and thus, have particle nature.
15. Which is massless among α , β and γ-rays?
Ans. γ-ray is massless.
16. Where does radioactive change take place inside an atom?
Ans. Radioactive change takes place in the nucleus of the atom.
17. Is a radioactive change unidirectional or bidirectional?
Ans. A radioactive change is always unidirectional.
18. A compound of a radioactive element is formed a chemical reaction. Is there any change does occur in radioactivity?
Ans. Radioactivity remains unchanged even if a compound of a radioactive element is formed in a chemical reaction.
19. What is the SI unit of radioactivity?
Ans. SI unit of radioactivity is becquerel (Bq).
20. The atomic number is not changed by which type of radioactive decay?
Ans. γ-decay.
21. Helium ions and a-particles are the same or different?
Ans. Helium ions and α -particles are the same.
22. What type of radioactive decay is caused when there are too many neutrons in the nucleus?
Ans. β-decay.
23. Which part of an atom undergoes a change in the process of radioactive decay?.
Ans. The nucleus of an atom undergoes a change in the process of radioactive decay.
24. Name the radioactive radiation which have the least penetrating power.
Ans. ‘α‘-radiation have the least penetrating power.
25. A radioactive substance is oxidised. What change takes place in the nature of radioactivity?
Ans. No change takes place.
26. Name the positively charged particle that emanates from an uranium nucleus.
Ans. The positively charged particles that emanates from an uranium nucleus is ‘α‘particle.
27. Name a negatively charged radioactive particle.
Ans. A negatively charged radioactive particle is β-particle.
28. Why are emissions of α -particles, β-particles and γ -rays called nuclear phenomenon?
Ans. These emissions are entirely nuclear phenomenon because that happens due to internal changes in the nucleus.
Fill in the blanks
1. γ-ray is a type of ………. wave.
Ans. electromagnetic
2. The atom of the radioactive element which undergoes radioactive decay is called ……… atom.
Ans. parent
3. The atom of the new element that is produced after emission of radioactive ray from the nucleus is called a ……… atom.
Ans. daughter
4. The rest mass of γ-ray is ………..
Ans. zero
5. The age of a fossil and an archaeological object is determined by measuring the ratio of …….. and 12C in it.
Ans. 14C
6. The charge of an α-particle is ………… of that of a hydrogen ion.
Ans. twice
7. If an α-ray falls on a screen coated with zinc sulphide, it creates ……….
Ans. fluorescence
8. α -particle is the nucleus of a ………… atom.
Ans. helium
9 Atomic number of a radioactive nucleus is not affected due to emission of ………… -ray from it.
Ans. γ
10. Charge of positron is …………
Ans. + 1.6 × 10-19C
11. Radioactivity is totally a ……….. phenomenon.
Ans. nuclear
TOPIC – B
Nuclear Energy
SHORT AND LONG ANSWER TYPE QUESTIONS
Q.1 What is nuclear reaction? Write down Einstein’s principle of equivalence of mass and energy.
Ans. Nuclear reaction is a process in which two nuclei, or else a nucleus of an atom and a subatomic particle from outside the atom, collide to produce one or more nuclides that are different from the initial nuclide(s).
According to Einstein’s theory of relativity, mass is concentrated energy. It is possible to convert mass into energy and energy into mass. If an amount of mass m of a substance is converted fully into energy, then an amount of energy E = mc2 is released where c = 2.998 × 108m/s = speed of light in vacuum.
Q.2 What is mass defect? Calculate the mass defect of the nucleus AZX with mass M. Given, the mass of a proton = mp and the mass of a neutron = mn.
Ans. The mass of a nucleus is slightly less than the mass of the sum of protons and neutrons present in the nucleus. This difference between the two masses is called the mass defect.
According to the above question, the atomic number and the mass number of the atom are Z and A, respectively. If the masses of the proton and the neutron are mp and mn, respectively and the mass of the nucleus of the atom is M, then total mass of the nucleons
= Z · mp + (A – Z)mn
∴ mass defect = Z · mp + (A – Z)mn – M
Q.3 What is binding energy? Calculate the binding energy of the nucleus AZX with mass M.Given the mass of a proton = mp and the mass of a neutron = mn.
Ans. Binding energy is defined as the minimum energy required to disassemble a nucleus of an atom into its constituent particles. It is the equivalent amount of energy of mass defect.
According to the above question, the atomic number and the mass number of the atom are Z and A, respectively. If the masses of the proton and the neutron are mp and mn, respectively and the mass of the nucleus of the atom is M, then total mass of the nucleons
= Z · mp + (A – Z)mn
∴ mass defect = Z · mp + (A – Z)mn – M
and hence binding energy
= {Z · mp + (A – Z)mn – M}c²
where c is the speed of light in vacuum.
Q.4 What do you mean by a chain reaction? What is a nuclear reactor?
Ans. In a nuclear fission due to bombardment of neutrons on each 235U atom, high speed neutrons are ejected from the reaction. These are called secondary neutrons. These secondary neutrons interact with the surrounding uranium nucleus. If sufficient fissile fuel is present, some of these neutrons may be absorbed and cause more fissions. Thus, the cycle repeats and the process of nuclear fission continues spontaneously. This type of spontaneous reaction is called a chain reaction.
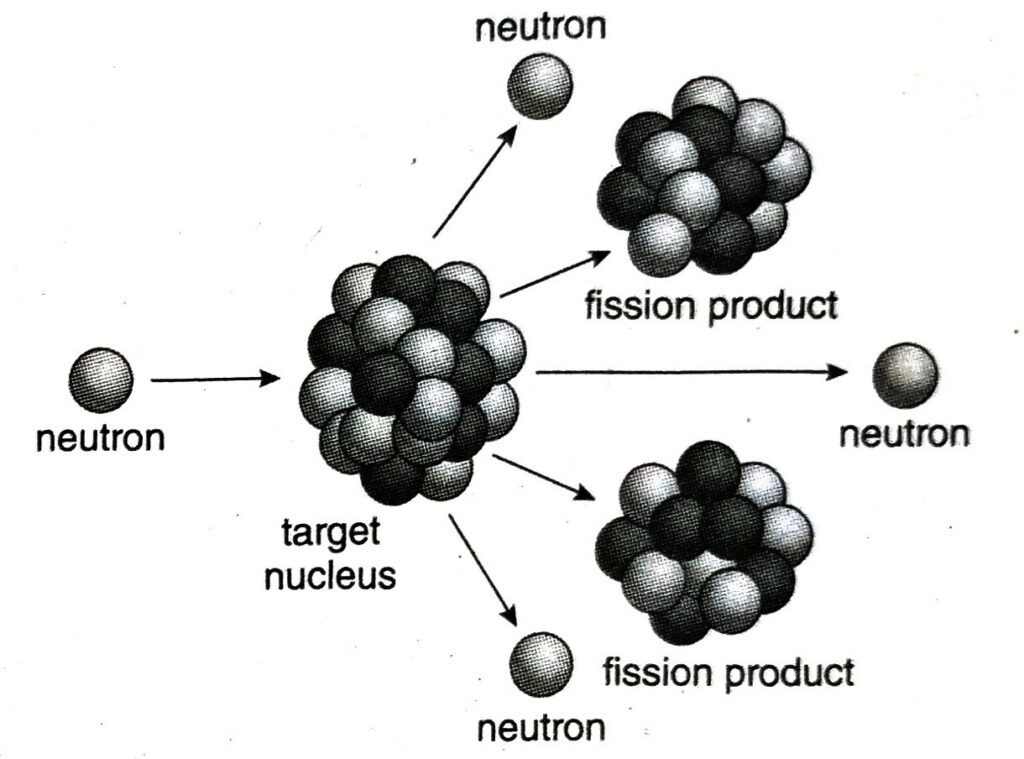
A nuclear reactor is an arrangement used to initiate and control a sustained nuclear chain reaction. Nuclear reactors are used at nuclear power plants mainly for electricity generation.
Q.5 Write down the names of some nonconventional sources of energy other than atomic energy. What are the advantages in production of atomic energy?
Ans. Some sources of non-conventional energy other than atomic energy are solar energy, wind energy, tidal energy etc.
Production of atomic energy is not affected by any natural event like cyclone, storm, rain, earthquake etc. Atomic energy can be produced day and night over all the year.
But this advantage is not there in case of production of other energies. This is the advantage of production of atomic energy.
Q.6 What are the advantages of atomic energy as a fuel compared to fossil fuel?
Ans. Advantages of atomic energy over energy from fossil fuel:
| Energy from fossil fuel | Atomic energy |
| 1. Due to rampant use of fossil fuels (like coal, petrol, diesel, kerosene etc.), amount of carbon dioxide and other Greenhouse gases is increasing in the atmosphere. | 1. Production of harmful gases reduce owing to the use of atomic energy. |
| 2. The amount of fossil fuel used to get a certain amount of energy is slowly pushing us towards a serious fuel crisis. This is a major problem at present for the human civilisation. | 2. A huge amount of energy is available due to nuclear fission from a very small amount of material. This is not possible from any other source and also is a great saviour compared to any other fuel. For example, nearly 3000 tons of coal have to be burnt to get the same amount of energy (nearly 7.4 × 1010J) that is available from fission of only 1 g of U-235. |
Q.7 Why is a fission reaction carried out before a fusion reaction? Several lacs of people were killed in Japan in 1945 due to the explosion of an atom bomb. In 1986, the nuclear reactor at Chernobyl of Ukraine went out of control and the residents of that town suffered nearly the same fate. Is there any difference in the scientific causes of these two incidents?
Ans. 107°C to 108° C temperature is required for fusion of the nuclei. This high temperature cannot be produced in a simple way. This high temperature can be produced by the process of nuclear fission only. Therefore, a fission process takes place before a fusion reaction.
The first incident was due to the bad intention of a class of warmongers. Due to uncontrolled nuclear fission, huge amount of heat energy is produced in this type of atomic explosion. Radioactive radiation is also emitted in it. Due to these two reasons, a lot of people were killed.

The second one was simply an undesirable accident. In a nuclear reactor, heat energy is converted into electrical energy by causing a controlled nuclear fission. In other words, atomic energy is utilised in a peaceful way through this process for the welfare of mankind. But unfortunately on 26th April, 1986, the process of nuclear fission in a nuclear reactor at Chernobyl of Ukraine went out of control and emitted huge amount of radioactive rays. As a result, the residents of the city became the victims of radioactivity for a long time.
Q.8 What are used as (1) Fuel, (2) Moderator, (3) Control rods and (4) Coolant in a nuclear reactor and what are their functions?
Ans.
| Name of the part | Main material used | Function |
| 1. Fuel | Uranium (radioactive element) [U-235] | U-235 is bombarded with thermal neutrons bring about fission |
| 2. Moderator | Heavy water (D2O) or graphite | Neutrons with high kinetic energy generated due to nuclear reactions are slowed down by it |
| 3. Control rods | Cadmium (Cd), hafnium (Hf) or boron (B) coated steel rods | To absorb excess thermal neutrons that are not required |
| 4. Coolant | Generally water | To absorb heat energy that is produced in the core due to nuclear reactions |
Q.9 What is nuclear fusion?
Calculate the amount of energy released in this process by taking 1u ≡ 931.2 MeV.
Ans. Nuclear fusion is a process in which two light

nuclei are fused or combined at a very high temperature to form a heavy nucleus. This process releases a large amount of energy.
Mass of 2 deuterons in the given equation before reaction,

Q.10 Write down the differences between nuclear fission and nuclear fusion.
Ans. The differences between nuclear fission and nuclear fusion are:
| Nuclear fission | Nuclear fusion |
| 1. A heavy nucleus is split into two nuclei of almost equal masses in this process. | 1. A few light nuclei are fused together to form a heavy nucleus in this process. |
| 2. The energy produced in this process is less than the energy produced by fusion of materials of equal masses. | 2. The energy produced in this process is more than the energy produced by fission of equal masses. |
| 3. Thermal neutrons are required in this process to bombard the target nucleus. | 3. No bombarding particle is required in this process. |
| 4. This process takes place at normal temperature. | 4. This process takes place at a temperature between 107°C and 108°C. |
| 5. Radioactive rays are emitted in this process. | 5. No radioactive rays are emitted in this process. |
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. The main cause of the solar energy is
A. nuclear fission and fusion
B. nuclear fission
C. nuclear fusion
D. chemical reaction
Ans. C
2. What is the average binding energy of each nucleon inside a nucleus to make the nucleus stable?
A. nearly 2 MeV
B. nearly 2.5 MeV
C. nearly 3 MeV
D. nearly 8 MeV
Ans. D
3. 1eV = how many J?
A. 3.2 × 10-19
B. 1.6 × 10-19
C. 4.8 × 10-19
A. 6.4 × 10-19
Ans. B
4. 1MeV = how many J?
A. 1.6 × 10-13
B. 3.2 × 10-13
C. 4.8 × 10-13
A. 6.4 × 10-13
Ans. A
5. Binding energy per nucleon is maximum in
A. 60Ni
B. 56Fe
C. 58Fe
D. 62Ni
Ans. D
6. Value of binding energy per nucleon of 62Ni is approximately
A. 8.0 MeV
B. 9.2 MeV
C. 8.8 MeV
D. 8.2 MeV
Ans. C
7. How much energy is produced if 0.1 u amount of mass is converted into energy?
A. 186.3 MeV
B. 46.575 MeV
C. 93.12 MeV
D. 372.6 MeV
Ans. C
8. Nuclear binding energy is equivalent to
A. mass of proton
B. mass of neutron
C. mass of nucleus
D. mass defect of nucleus
Ans. D
9. Average binding energy per nucleon in the nucleus of an atom is approximately
A. 8 eV
B. 8 keV
C. 8 MeV
D. 8J
Ans. C
10. A moderator is used in nuclear reactor in order to
A. slow down the speed of the neutrons
B. accelerate the neutrons
C. increase the number of neutrons
D. decrease the number of neutrons
Ans. A
11. The control rod in a nuclear reactor is made of
A. uranium
B. cadmium
C. plutonium
D. graphite
Ans. B
12. The process by which a heavy nucleus splits into light nuclei is known as
A. fission
B. fusion
C. chain reaction
D. meltdown
Ans. A
13. During the nuclear fusion reaction
A. a heavy nucleus breaks into two fragments by itself
B. a light nucleus bombarded by thermal neutrons breaks up
C. a heavy nucleus bombarded by thermal neutrons breaks up
D. two light nuclei combine to give a heavier nucleus and possible other products
Ans. D
14. Fusion reaction takes place at high temperature because
A. atoms are ionised at high temperature
B. molecules break up at high temperature
C. nuclei break up at high temperature
D. kinetic energy is high enough to overcome repulsion between nuclei
Ans. D
15. The explosion of the atomic bomb takes place due to
A. nuclear fission
B. nuclear fusion
C. melting
D. evaporation
Ans. A
16. A chain reaction continues due to
A. large mass defect
B. large energy
C. production of more neutrons in fission
D. high temperature
Ans. C
17. Heavy water (D2O) is used as moderator in a nuclear reactor. The function of the moderator is
A. to control the energy released in the reactor
B. to absorb neutrons and stop chain reaction
C. to cool the reactor fast
D. to slow down the speed of neutrons
Ans. D
Answer in brief
1. What is the temperature required to bring about nuclear fusion?
Ans. Nuclear fusion takes place at a temperature of 107°C to 108°C.
2. What is the main source of energy for the sun and the stars?
Ans. Nuclear fusion is the main source of energy for the sun and the stars.
3. Name one radioactive material which is much used as a fuel in a nuclear reactor.
Ans. Uranium, a radioactive material, is generally used as a fuel in a nuclear reactor.
4. What is the role of heavy water in a nuclear reactor?
Ans. Heavy water is used in a nuclear reactor to slow down the speed of neutrons.
5. Binding energy of 42He nucleus is 28.2 MeV. What do you understand by this statement?
Ans. The given statement means that a minimum energy of 28.2 MeV is required to separate 2 protons and 2 neutrons from a helium nucleus to make them free.
6. Which element has the highest binding energy per nucleon?
Ans. 62Ni has the highest binding energy per nucleon.
7. What do you understand by mass defect?
Ans. The mass of a nucleus is slightly less than the mass of the sum of protons and neutrons present in the nucleus, this difference of two masses is called the mass defect.
8. What is binding energy of a nucleus?
Ans. Nuclear binding energy is the minimum energy that is required to disassemble the nucleus of an atom into its component parts, i.e., neutrons and protons.
9. Who did invent the formula of equivalence of mass and energy?
Ans. Albert Einstein invented the formula.
10. Write down Einstein’s formula for massenergy equivalence.
Ans. Formula: E= mc², where m = mass of material, c = speed of light in vacuum and E is the amount of energy equivalent to mass m.
11. What is nuclear fission?
Ans. Nuclear fission is the process of splitting of a heavy nucleus into two lighter nuclei of comparable masses with liberation of energy.
12. What is the main source of energy gained in a nuclear fusion or fission?
Ans. The main source of energy gained in a nuclear fusion or fission is decrease or reduction in mass.
13. Which nuclear process was followed to manufacture an atom bomb?
Ans. Nuclear fission was followed to manufacture an atom bomb.
14. Which particle is used as a bombarding particle to bombard the target nucleus in the process of nuclear fission?
Ans. Neutron is used as a bombarding particle.
15. How much energy is produced if 1 u amount of mass is converted into energy?
Ans. 931.2 MeV (approx.) energy is produced if 1u amount of mass is converted into energy.
16. What is a thermal neutron?
Ans. A neutron with a high kinetic energy of about 10-2eV is called thermal neutron.
17. Write down an use of thermal neutron.
Ans. Thermal neutron is used to split a heavy nucleus into two or more lighter nuclei in nuclear fission.
18. What is the relation between mass defect and nuclear binding energy?
Ans. Nuclear binding energy is energy equivalent to the mass defect of a nucleus.
19. Is the law of conservation of mass applicable in case of nuclear reaction?
Ans. In case of nuclear reaction the law of conservation of mass is not applicable.
20. Is the law of conservation of mass number and the law of conservation of atomic number applicable in case of nuclear reaction?
Ans. Yes, the law of conservation of mass number and the law of conservation of atomic number are applicable in case of nuclear reaction.
21. What is nuclear reactor?
Ans. A nuclear reactor is an arrangement used to initiate and control a sustained nuclear chain reaction.
22. What is the role of moderator in a nuclear reactor?
Ans. Moderator is used to slow down the speed of neutrons.
23. What is the role of control rod in a nuclear reactor?
Ans. The control rod absorbs surplus thermal neutrons.
24. What material generally used as control rod in nuclear reactor?
Ans. Cadmium rod or steel rod with a coating of boron is generally used as control rod.
Fill in the blanks
1. The mass of a nucleus is slightly …………. than the sum of the masses of neutrons and protons present in that nucleus.
Ans. less
2. The process of nuclear …………. is carried out before nuclear fusion takes place.
Ans. fission
3. Mass of the nucleus of deuterium (21H) is ………… than the sum of masses of one proton and one neutron.
Ans. less
4. Chain reaction is observed in nuclear…………
Ans. fission
5. The main cause in producing energy for sun is nuclear ………
Ans. fusion
6. Fusion process occurs at a very high temperature. Such a process is called ……….. reaction.
Ans. thermonuclear
7. ………… neutron is used as projectile 7 Salam particle in nuclear fission reaction.
Ans. Thermal/Slow
8. Heavy water (D2O) is used as ………… in nuclear reactor.
Ans. moderator
9. The greater is the binding energy the more …………. is the nucleus.
Ans. stable
State whether true or false
1. High energy neutrons are used in a nuclear reactor to initiate the fission reaction.
Ans. False
2. Cadmium rods are used as moderators in a nuclear reactor.
Ans. False
3. Higher the mass defect, higher is the stability of the nucleus.
Ans. False
5. The conversion of 1u of mass results in 931.2 × 106eV of energy.
Ans. True
6. In sun and other stars, the energy is produced by nuclear fusion.
Ans. True
7. Mass defect is always positive.
Ans. True
8. Electron is used as an ideal particle for bombarding.
Ans. False
9. Hydrogen bomb is made on the basis of nuclear fission reaction.
Ans. False
10. The Fukushima nuclear plant disaster was a nuclear accident caused by a natural disaster-tsunami.
Ans. True
Follow on Facebook page – Click Here
Google News join in – Click Here
Read More Asia News – Click Here
Read More Sports News – Click Here
Read More Crypto News – Click Here