WBBSE 10th Class Science Solutions Physical Science & Environment Chapter 8.1 Periodic Table and Periodicity in the Properties of Elements
West Bengal Board 10th Class Science Solutions Physical Science & Environment Chapter 8.1 Periodic Table and Periodicity in the Properties of Elements
WBBSE 10th Class Physical Science & Environment Solutions
8.1 Periodic Table and Periodicity in the Properties of Elements
Synopsis
Periodic Table
- Dobereiner’s Law of Triads: In 1817, German scientist Dobereiner first attempted to arrange the elements on the basis of their atomic weights. According to his Law of Triads, if three elements having similar properties are arranged in increasing order of their atomic weights, the atomic weight of the middle element in the triad is found to be the average of the atomic weights of the remaining two elements.
- Newland’s Law of Octaves: When the known elements are arranged in increasing order of their atomic weights, the eighth element, starting from a given one, will show resemblance in physical and chemical properties with the first element. This is known as Newland’s Law of Octaves (1861).
- Mendeleev’s Periodic Law: In 1869, Mendeleev put forward the periodic law through which he established the relationship between atomic weight of an element to its physical and, chemical properties. The statement of the law was given as-
The physical and chemical properties of the elements are periodic function of their atomic weights.
Almost at the same time, German scientist Lothar Meyer came to the same conclusion by observing that physical properties of the elements are changing periodically with their atomic weights.
- Periodic Table: On the basis of his periodic law, Mendeleev constructed a table by arranging the elements in increasing order of their atomic weights. This table is known as Mendeleev’s periodic table.
- Modern Periodic Law: In 1913, British scientist Moseley while observing the effects of X-rays on metals, concluded that atomic number is a more accurate and fundamental property of an element than its atomic weight. Accordingly Mendeleev’s periodic law was modified. This modified form is known as the modern periodic law which states that-
The physical and chemical properties of the elements are the periodic functions of their atomic number
- In the periodic table, the horizontal rows of elements are called periods and the vertical columns of elements are called groups. The modified version of Mendeleev’s periodic table has 7 periods and 9 groups.
- Long form of periodic table or Modern periodic table: The periodic table constructed on the basis of electronic. configuration of elements is known as long form of periodic table or the modern periodic table. It is also known as Bohr’s periodic table. It consists of 7 periods and 18 groups.
TOPIC – A
Periodic Table
SHORT AND LONG ANSWER TYPE QUESTIONS
Q.1 State Dobereiner’s ‘Law of Triads’? Why was the law discarded?
Ans. If the elements of a triad i.e., a group of three elements having similar chemical properties, are arranged in increasing order of their atomic weights, then the atomic weight of the middle element is found to be equal or almost equal to the average of atomic weights of the other two elements..
Example: Li (7), Na (23) and K (39) have similar chemical properties and thus forms a triad. The average of atomic weights of Li and K = 7+39/2 = 23, which is the atomic weight of Na.
The law failed to arrange most of the elements known till then. It was only applicable for few elements. For these reasons, the law was discarded.
Q.2 State the Mendeleev’s periodic law. What was the basis of Mendeleev’s periodic law?
Ans. The physical and chemical properties elements are periodic function of their atomic weights.
Mendeleev examined the relationships between the atomic masses of the elements and their physical and chemical properties such as boiling point, melting point, density, atomic mass, formula of hydrides and oxides of the elements etc. He observed that the physical and chemical properties of the elements change and periodically repeat with the change in atomic masses. Based on these observations, he arranged the elements in the order of increasing atomic masses and formulated the periodic law.
Q.3 Evaluate the contribution of Lothar Meyer in periodic classification of elements.
Ans. German scientist Lothar Meyer (1870) worked extensively on the relationship between physical properties of elements (such as atomic volume, melting point, boiling point etc.) and their atomic weights. He also stated a periodic law which was quite similar to what Mendeleev had proposed.
The work of both Mendeleev and Lothar Meyer are acknowledged towards periodic classification of elements. However, Mendeleev got greater recognition in this field as he put forward the theory much earlier than Lothar Meyer. However, the contribution of Lothar Meyer towards the concept of periodicity and periodic classification of elements cannot be neglected.
Q.4 What do you mean by period and group of the periodic table? How many periods and groups were present in the Mendeleev’s periodic table published in 1871?
Ans. The horizontal arrays of periodic table are termed as periods and the vertical arrays are termed as groups of the periodic table.
There were 7 periods (1, 2, 3,…, 7) and 8 groups (I-VIII) in the periodic table published in 1871.
Q.5 How many elements were mentioned in the main version of Mendeleev’s periodic table published in 1871? Which element was absent in the table and why?
Ans. 63 elements were mentioned in the Mendeleev’s periodic table published in 1871.
Group ‘0’ (zero) was absent in the Mendeleev’s periodic table (1871) as inert gases were not discovered at that time.
Q.6 Why were subgroups needed in the eded in Mendeleev’s periodic table?
Ans. In Mendeleev’s periodic table it was found that except group ¹0 (zero) and group. VIII, several elements of different properties are placed in the same group. Each of the group except 0 and VIII is divided into two subgroups designated as ‘A’ and ‘B’. Properties of the elements of subgroup A and B are altogether different, except their valencies. However, elements of the same subgroup exhibit more or less similar properties. E.g., the alkali metals of Group I-A are closely alike. But Group IA metals differ remarkably from the coinage metals of Group IB, although they have a common valency of ‘1’.
Q.7 Atomic mass of argon (39.94) is greater than that of potassium (39.1), yet potassium was placed after Argon in Mendeleev’s periodic table-explain why.
Ans. Mendeleev’s main objective was to place elements With similar properties in the same class. That is why these two elements were placed in the group where their properties resemble with other elements although their atomic masses indicates just the reverse places in the periodic table.
Q.8 How was was Mendeleev’s periodic table useful in the periodic classification of elements? Explain with example.
Ans. The elements present in the same group of the periodic table show similarity in properties.
So, if the property of one element in a specific group is known we can get an idea about properties of the other elements present in the group.
If we know the properties of sodium of groupIA, the properties of other elements of that group namely potassium, rubidium and caesium can easily be evaluated.
Q.9 How was Mendeleev’s periodic table helpful in describing the structure and electronic configuration of different elements?
Ans. The elements present in the same subgroup of a group in Mendeleev’s periodic table have similar physical and chemical properties. Again, it is known that elements with similar properties have similar electronic configuration of their outermost orbits. Thus, it can easily be correlated that elements of the same group have similar electronic configuration. Therefore, we can get an idea about the electronic configuration of other elements of a definite group, if the electronic configuration of any one element of the group is known. Similarly, from the knowledge of electronic configuration, we get an idea about the structure of atom.
Q.10 Discuss the limitations of Mendeleev’s periodic table.
Ans. Though Mendeleev was almost successful in arranging the elements on the basis of their properties in his periodic table, the periodic table had some serious drawbacks such as-
- In some cases, in order to place the elements having similar properties in the same group, Mendeleev placed some elements with higher atomic weight before elements having lower atomic weight.
- The position of hydrogen in Mendeleev’s periodic table was controversial.
- In some cases, Mendeleev put ‘elements having similar properties in different groups while elements having different properties in the same group.
- Though the atomic weights of different isotopes are different, they were not given different positions in the periodic table.
Q.11 Mention the observation and inference of Moseley’s experiment on X-ray spectra.
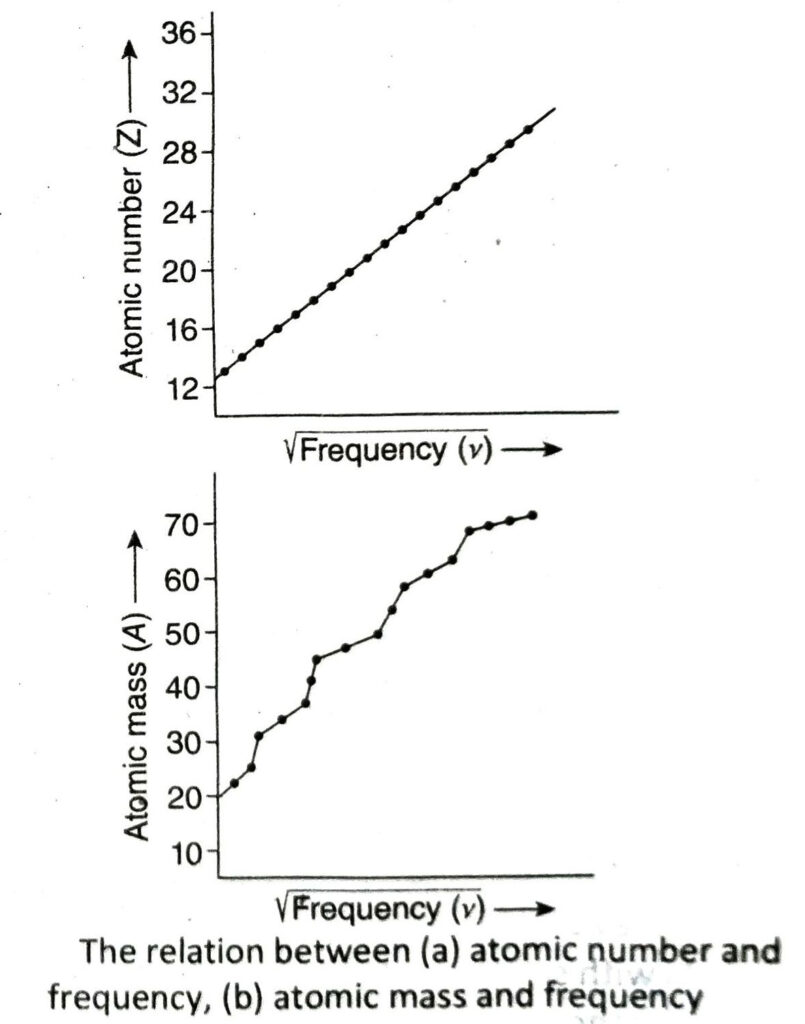
Ans. In 1913, scientist Moseley made extensive research on the X-rays produced when cathode rays are bombarded on plates (target or anticathode) made of different metals.
Observation: (1) He observed that the X-rays produced due to collision of cathode rays with different metals have different frequencies. However, for a definite metal the frequency of produced X-rays is constant. (2) He also observed when the square root of the frequencies of radiated X-rays were plotted against the atomic numbers of the elements (metals) used as anticathode, a straight line was obtained. However, no such straight line was obtained when atomic masses of the elements were ploted against the square root of the frequencies.
Inference: From the experiment, Moseley inferred that the properties of elements are a function of their atomic numbers and not their atomic masses.
Q.12 Write down the modified or modern periodic law. Mention the total number of periods and groups in the modern edition of Mendeleev’s periodic table.
Ans. The physical and chemical properties of the elements are a periodic function of their atomic numbers.
There are 7 periods (1-7) and 9 groups (I, VIII and 0) in the modern edition of Mendeleev’s periodic table.
Q.13 The chemical properties of all the elements in the same period are not the same, but the chemical properties of the elements in the same group are similar-explain.
Ans. The chemical properties of the elements depend on the atomic number and hence on the electronic configuration of the atoms. Since the atomic number and electronic configuration of the elements in a particular period are different, their chemical properties are also different.
On the other hand, although the atomic numbers of different elements belonging to the same group are different, the electronic configurations of the outermost shell are similar. That is why their chemical properties are also similar to each other.
Q.14 Why are the elements arranged in increasing order of their atomic number in the long form of periodic table, instead of their increasing atomic weight?
Ans. In 1913-14, British scientist Moseley, on the basis of his experiments on X-ray spectra of different atoms, explained that atomic number is a more fundamental property of an element than its atomic weight. It controls the physical and chemical properties of the elements. Again, after discovery of isotopes it was well known that an element may contain atoms of different atomic weight. Thus, it was proved that atomic weight does not control the properties of an element. So, in the long form of periodic table, elements are arranged in increasing order of their atomic number instead of atomic weight.
Q.15 Which period is termed as the shortest period? Mention the groups where the elements of the shortest period reside.
Ans. The 1st period of the periodic table is termed as the shortest period. It contains only two elements.
Among the two elements of the 1st period, Hydrogen belongs to group-1 and helium belongs to group-18 of the periodic table.
Q.16. What is meant by ‘representative elements’?
Ans. The elements of 2nd and 3rd periods are abundant in nature. Apart from this, the elements of a certain group in these periods show much resemblance in properties in regular intervals. So, these elements are collectively called ‘typical or representative elements’
[Apart from these elements, 8 elements from 4th period- K(19), Ca(20) and Ga(31) to Kr(36); 8 elements from 5th period – Rb(37), Sr(38) and In(49) to Xe(54); 8 elements from 6th periodCs(55), Ba(56) and Th(81) to Rn (86) and two elements from 7th period – Fr(87) and Ra(88) are also known as representative elements.]
Q. 17 What are alkali metals and alkaline earth metals? Why are they called so?
Ans. The elements present in group-IA or group-1 of the periodic table, i.e., lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs) and francium (Fr) are collectively called alkali metals. The oxides, hydroxide and carbonates of these metals are strongly alkaline in nature. So, they are called alkali metals.
The elements present in group-IIA or group-2 of the periodic table, i.e., beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba) and radium (Ra) are collectively called alkaline earth metals.
Compounds of these elements are abundantly found in the earth’s crust and their oxides, hydroxides and carbonates are alkaline in nature. So, they are called alkaline earth metals.
Q.18 Write down the characteristics of alkali metals.
Ans. Characteristics of alkali metals are-
- Alkali metals are highly electropositive and they have a tendency to form cation by losing electron from their valence shell.
- Because of high reactivity these elements do not occur freely in nature.
- Valence shell of alkali metals contain 1 electron and thus alkali metals are monovalent.
- Oxides and hydroxides of alkali metals are strong bases and alkalies respectively.
- Alkali metals form metallic hydroxides and hydrogen in reaction with water.
- Alkali metals are good reducing agents. Ascending order of reducing power of alkali metals is – Li < Na < K < Rb < Cs.
Q.19 What are bridge they called so? elements? Why are bridge elements?
Ans. The inert gases (He, Ne, Ar, Kr, Xe and Rn) present in group-0 or group-18 of the periodic table are known as bridge elements.
These elements act as bridge between strong electronegative halogens of group-17 and strong electropositive alkali metals of group-1 of the next period. So, these elements are called bridge elements.
Q.20 What are transition elements?
Ans. The elements from scandium (Sc) to copper (Cu) in the fourth period, yttrium (Y) to silver in the 5th period, lanthanum (La) and hafnium (Hf) to gold (Au) in the 6th period are known as transition elements due to some specific characteristics in their electronic configurations.
Q.21 Write down some characteristics or properties of transition elements.
Ans.
- Except mercury (Hg), all transition elements are solid metals.
- Transition elements generally exhibit variable oxidation states and valencies.
- They can form both ionic and covalent compounds.
- Transition elements exhibit a very distinctive property of forming coloured co-ordination complexes.
- A number of transition metals such as Cr, Mn, Fe, Co, Ni, Cu etc. and their compounds are used as catalysts.
- Many transition metals form alloys.
Q.22 which elements are known as pnictogens? Why are they named so?
Ans. The elements of group V B of Mendeleev’s periodic table or group 15 of the long form of periodic table are known as pnictogens. E.g., nitrogen (N), phosphorous (P), arsenic (As), antimony (Sb), bismuth (Bi).
The word pnictogen is derived from the Greek word pnicogens meaning ‘suffocating’ or ‘to choke’. The first member of group 15 is nitrogen which is a suffocating gas. That is why members of this group are known as pnictogens.
Q.23 What are chalcogens? Why are they are chalcogens? Why are called so?
Ans. The elements of group-16 of the periodic table namely O, S, Se, Te and Po are collectively known as chalcogens.
The term ‘chalcogen’ means producer of ores. Most of the metals are found as their oxide or sulphide ores in earth’s crust. So, these elements are known as chalcogens.
Q.24 What are metalloids? Give example.
Ans. There are some elements which have certain characteristics common to both metals and nonmetals. These are called metalloids.
Example of metalloids are arsenic (As), antimony (Sb), bismuth (Bi).
Q.25 Isotopes of an element have not been given separate places in the periodic table- this is one of the major drawbacks of Mendeleev’s periodic table. Explain.
Ans. In Mendeleev’s periodic table, the elements were arranged in increasing order of their atomic weights. However, the isotopes of an element have different atomic weights. But, Mendeleev placed all the isotopes of an element in the same group and same period which violates the basic rule of periodic classification. So, it can be considered as a major drawback of Mendeleev’s periodic table.
But in modern periodic table, the elements are arranged in increasing order of their atomic numbers. As all the isotopes of an element have the same atomic number, they should be positioned in the same place in the periodic table according to modern periodic law. Hence, this is no longer a drawback in modern periodic table.
Q.26 Discuss three advantages of modern periodic table over Mendeleev’s periodic table.
Ans. Three advantages of long form of periodic table over Mendeleev’s periodic table are discussed below-
- In Mendeleev’s periodic table the elements were arranged in increasing order of their atomic weights and later in increasing order of their atomic numbers. But in long form of periodic table, the elements are arranged on the basis of their electronic configurations. Elements having same electronic configuration in their outermost shell are placed in the same group. As the chemical properties are related to the electronic configuration of the outermost shell, the periodicity of properties of the elements are described more effectively in long form of periodic table.
- In Mendeleev’s periodic table, each group except group-VIII and group-0, was divided into subgroups. But the elements of different subgroups of a definite group are not very similar in terms of their properties. In modern periodic table, the concepts of subgroups has been eliminated. Elements of different subgroups have been placed in completely different groups. Which is more scientific.
- In Mendeleev’s periodic table, each period of group-VIII had three elements, namely, (Fe, Co, Ni), (Ru, Rh, Pd) and (Os, Ir, Pt). This defied the basic rule of periodic classification. In the long form of periodic table, these elements are placed in different groups.
Q.27 which elements are halogens? Why are these named so?
Ans. Elements of Gr-17 in the modern periodic table i.e. F, Cl, Br, I, At etc. are termed as halogens.
The name ‘halogen’ means ‘salt-producing’. Several salts can be formed by the reaction of metal with these elements. Salts containing F, Cl or I have been found in sea water. That is why these elements are termed as halogen. Some salts of halogen are-NaCl (common salt), CaF2, NaBr, Kl etc.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. Which of the following sets of elements does not form a triad?
A. Ca, Sr, Ba
B. Li, Na, K
C. O, S, Se
D. Cl, Br, I
Ans. C
2. The number of elements in the longest period and the incomplete period are respectively
A. 28, 32
B. 32, 28
C. 32, 32
D. 18, 28
Ans. B
3. The elements that found no place in the original periodic table constructed by Mendeleev were
A. transition metals
B. alkali metals
C. noble gases
D. halogens
Ans. C
4. In Mendeleev’s periodic table, the arrangement of elements in the order of their increasing atomic weights was not maintained for which pair of elements?
A. C & N
B. S & Cl
C. K & Ar
D. K & Ca
Ans. C
5. The group having no subgroups in Mendeleev’s periodic table is
A. group II
B. group VIII
C. group VII
D. group V
Ans. B
6. Which group in Mendeleev’s period table contains more than one element in the same position?
A. group II
B. group III
C. group 0
D. group VIII
Ans. D
7. The number of elements in each position under group VIII is
A. 4
B. 5
C. 3
D. 2
Ans. C
8. The pair of elements belonging to pnictogens is
A. N, P
B.P, O
C. F, Cl
D. Na, K
Ans. A
9. Number of groups in the long form of periodic table is
A. 9
B. 12
C. 18
D. 20
Ans. C
10. In long form of periodic table, the elements are arranged on the basis of their
A. atomic mass
B. atomic number
C. electronic configuration
D. number of neutrons
Ans. C
11. In long form of periodic table, the alkali metals are placed in group
A. 1
B. 2
C. 3
D. 4
Ans. A
12. In long form of periodic table, the alkaline earth metals are placed in group
A. 1
B. 2
C. 3
D. none of these
Ans. B
13. In long form of periodic are placed in group table, the halogens
A. 15
B. 16
C. 17
D. 18
Ans. C
14. In long form of periodic table, the coinage metals are placed in group.
A. 11
B. 15
C. 17
D. 1
Ans. A
15. In long form of periodic table, the chalcogens are placed in group
A. 16
B. 17
C. 13
D. 1
Ans. A
16. Which of the following is a chalcogen?
A. Se
B. B
C. C
D. Ne
Ans. A
17. In long form of periodic table, the noble gases are placed in group
A. 17
B. 18
C. 16
D. 15
Ans. B
18. An example of a transition metal is
A. Ca
B. Mg
C. Fe
D. Al
Ans. C
19. In Mendeleev’s periodic table hydrogen can be placed
A. with halogens or with alkali metals
B. with chalcogens or with alkaline earth metals
C. with pnictogens or with coinage metals
D. with pnictogens or with alkaline earth metals
Ans. A
20. Which of the following is a coinage metal?
A. iron
B. silver
C. aluminium
D. nickel
Ans. B
21. A trans-uranium element is
A. Np
B. Ce
C. Lu
D. La
Ans. A
22. The outermost shell of second period elements is
A. K-shell
B. L-shell
C. M-shell
D. N-shell
Ans. B
23. Which of the following pair is not correct?
A. coinage metal : Cu
B. halogen : I
C. alkaline earth metal: Fe
D. inert element : Ne
Ans. C
24. Which one of the following is an alkali earth metal?
A. Rb
B. Ba
C. Sb
D. F
Ans. B
Answer in brief
1. The atomic weight of sodium is the average of atomic weights of lithium and potassium. What can be inferred from this using the law of triads?
Ans. Using the law of triads, it can be inferred that the chemical properties of lithium, sodium and potassium are similar.
2. Three elements A, B, and C obey Dobereiner’s law of triads. If the atomic weights of A and C are respectively 7 and 39, then find the atomic weight of B.
Ans. According to law of triads, the atomic weight of the middle element of the triad is the average of the atomic weights of the first and third element.
Hence, atomic weight of B
3. Newland’s law of octave is not applicable for which type of elements?
Ans. Newland’s law of octave is not applicable for heavy elements.
4. On which basis did Mendeleev arrange the elements in the period table?
Ans. Mendeleev arranged the elements in the periodic table in increasing order of their atomic weights.
5. How many groups in Mendeleev’s periodic table were divided into subgroups A & B?
Ans. In Mendeleev’s periodic table, seven (7) groups were divided into subgroups A & B.
6. Which groups in the Mendeleev’s periodic table (corrected version) do not contain any subgroup?
Ans. Zero (0) and Eighth (VIII) group.
7. How many more elements are there in the second period than the first period of the Mendeleev’s periodic table?
Ans. There are 6 more elements present in the 2nd period than the 1st period of the Mendeleev’s periodic table.
8. The initial form of Mendeleev’s periodic table does not contain noble gases. Why?
Ans. At the time when Mendeleev first published the periodic table, the noble gases were not discovered yet. So, the noble gases did not find any place in the initial form of Mendeleev’s periodic table.
9. What is the position of noble gases in modern version of Mendeleev’s periodic table?
Ans. In modern version of Mendeleev’s periodic table, the noble gases are placed in ‘zero’ (0) group.
10. The atomic masses of some elements were erroneous when Mendeleev constructed the periodic table. Why?
Ans. Valencies of the elements were incorrectly measured. These inaccurate valencies of elements were responsible for the erroneous values of atomic masses of some elements.
11. Which element was predicted as eka-silicon by Mendeleev?
Ans. The element predicted by Mendeleev as eka-silicon is now known as germanium.
12. Which element was predicted by Mendeleev as eka-aluminium?
Ans. Gallium was predicted by Mendeleev as eka-aluminium.
13. What similarity is observed between Na and Cu regarding their positions in periodic table?
Ans. Both Na and Cu were placed in group-1. Na was in group-IA and Cu was in group-IB.
14. Which is the most electropositive nonradioactive alkali metal?
Ans. Caesium (Cs) is the most electropositive nonradioactive alkali metal.
15. Which alkali metal is radioactive?
Ans. Francium (Fr) is a radioactive alkali metal.
16. Name an alkali metal which is a liquid at 30°C.
Ans. Caesium is a liquid at 30°C
17. Which alkaline earth metal is the heaviest?
Ans. Radium is the heaviest alkaline earth metal.
18. Which alkaline earth metal is the lightest?
Ans. Beryllium is the lightest alkaline earth metal.
19. Which is the radioactive halogen?
Ans. The radioactive halogen is astatine (At).
20. Which is the lightest halogen?
Ans. Fluorine is the lightest halogen.
21. Name a halogen that shows reducing property.
Ans. lodine shows reducing property.
22. Which group in the periodic table contains elements in all the three states, namely solid, liquid and gas?
Ans. Group-17 (group-VIIB) of the periodic table contains elements in all the three states. Fluorine and chlorine are gases, bromine is liquid and iodine is solid at normal temperature.
23. Which property is similar in all transuranium elements?
Ans. All the trans-uranium elements are radioactive elements.
24. Mention the period in which all the elements are radioactive.
Ans. The elements of the 7th period are all radioactive.
25. Name a radioactive noble gas.
Ans. A radioactive noble gas is radon.
26. What are pnictogens?
Ans. The elements of group-15 in the long form of periodic table are known as pictogens.
27. Name the noble gas present in 2nd period of the periodic table.
Ans. Neon (Ne) is the noble gas present in 2nd period of the periodic table.
28. Name the inert gas residing at the 2nd period of the long form of periodic table.
Ans. Neon (Ne).
29. State whether the transition elements are metals or non-metals.
Ans. Transition elements are metals.
30. Which transition element is a liquid metal?
Ans. Mercury (Hg).
31. Give example of noble metal.
Ans. Platinum (Pt).
32. Elements of which group of the periodic table is termed as chalcogens?
Ans. Group-16 elements are termed as chalcogens.
33. Mention the group of the rare earth elements in the periodic table.
Ans. Rare earth elements are present in the 3rd group of the modern periodic table.
34. Name the lanthanide element of lowest atomic number.
Ans. Cerium (Ce) of atomic number 58.
35. Mention two elements of the periodic table which are formed in laboratory.
Ans. Neptunium (Np, Atomic no: 93) and nobelium (No, atomic no: 102)
Fill in the blanks
1. Cl, ………… and I are members of a triad.
Ans. Br
2. Noble gases are placed at extreme ………. of the periodic table.
Ans. right
3. In modern periodic table, the elements are arranged on the basis of their ………. instead of ……….
Ans. atomic numbers, atomic weights
4. Moseley from his X-ray experiment came to the conclusion that ………. is the fundamental property of elements.
Ans. atomic number
5. The …………. period of periodic table is an incomplete period.
Ans. 7th
6. The elements in zero group are chemically …………
Ans. inert
7. Hydrogen shows similar properties with …………. as well as …………
Ans. alkali metals, halogens
8. Trans-uranium elements starts from the element with atomic number ………..
Ans. 93
9. There is no …………. in the long form of periodic table.
Ans. subgroup
10. The elements of A and B subgroups in a given group of Mendeleev’s periodic table show no similarity except in their ……….
Ans. valencies
11. The elements sodium, potassium etc., are called alkali metals because the ………. and ………. of these elements are strong bases.
Ans. oxides, hydroxides
12. Among the halogens, …………. is liquid at normal temperature.
Ans. bromine
State whether true or false
1. Atomic weight of Na is the average of the atomic weights of Li and K.
Ans. True
2. The law of octaves was found to be satisfactory in case of light elements.
Ans. True
3. The physical and chemical properties of the elements are the periodic functions of their atomic weights.
Ans. False
4. The elements after uranium are called transition elements.
Ans. False
5. Except H, all other elements in group-1 are reactive metals.
Ans. True
6. The initial form of Mendeleev’s periodic table does not contain noble gases.
Ans. True
7. Beryllium is the lightest metal among the alkaline earth metals.
Ans. True
8. All the group-17 elements in the periodic table exists in gaseous state at normal temperature.
Ans. False
9. Cu, Ag and Au are known as coinage metals.
Ans. True
10. Zn is a transition element.
Ans. False
11. Ne (neon) is the most electronegative element.
Ans. False
12. Law of Triads was given by scientist Lothar Meyer.
Ans. False
13. There was no subgroup in group VIII of the Mendeleev’s periodic table.
Ans. True
14. In group VIB of Mendeleev’s periodic table elements of all three states viz. solid, liquid, gas are present.
Ans. False
15. Halogens are the member of Group zero (0) of the modern periodic table.
Ans. False
16. Halogen means sea-salt producer.
Ans. True
17. Number of transition elements in the 4th period is 10.
Ans. True
TOPIC – B
Chemical Periodicity of Elements
SHORT AND LONG ANSWER TYPE QUESTIONS
Q.1 What is meant by ‘periodic properties’?
Ans. With the increase in atomic number, physical and chemical properties of the elements belonging to different periods in the periodic table show a gradual change and as we move along a period, the change is systemic and periodic. Similarly, the elements present in a group show periodic variation of the similar properties with the increase in atomic number down the group. The physical and chemical properties of elements which show regular and periodic change across a period and down a group are known as periodic properties. Atomic radius, ionisation energy, electronegativity, oxidising and reducing property etc., are some of the common periodic properties.
Q.2 Define atomic radius.
Ans. The distance from the centre of the nucleus to the outermost shell containing the electrons is called atomic radius.
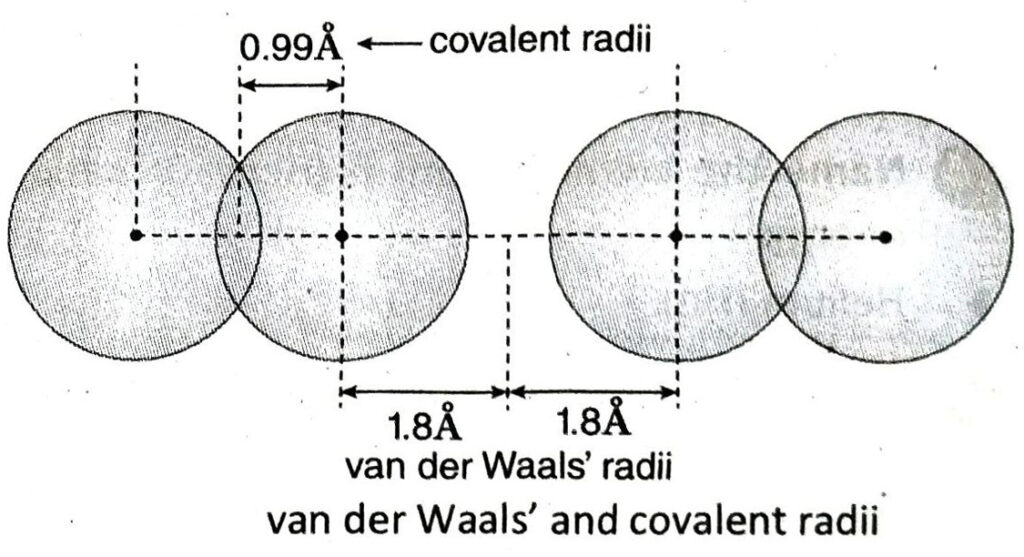
Since it is not possible to isolate a single atom for the measurement of its radius, atomic radius is expressed as covalent radius, metallic radius and van der Waal’s radius.
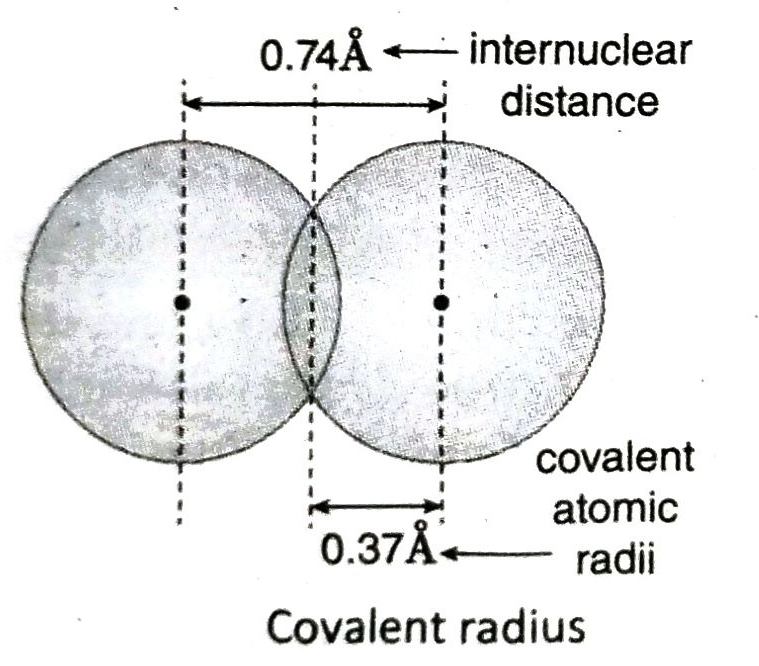
Q.3 Define covalent radius. If the internuclear distance of the two atoms in a hydrogen molecule is 0.74Å, then find its covalent radius.
Ans. When two similar atoms are connected to each other by a single bond, one half of the distance between their nuclei is called covalent radius.
If the internuclear distance of the two atoms in a hydrogen molecule is 0.74 Å, the covalent
radius of hydrogen will be = 0.74/2Å = 0.37 Å. A
Q.4 How does atomic radius of the elements change from left to right in a period? Give reason.
Ans. While moving from left to right across a period in the periodic table, the atomic radii or sizes of the atoms progressively decrease.
The principal quantum shell remains unchanged in the same period. So, the differentiating electrons enter the same shell. But due to increase in the number of protons the value of +ve charge of nucleus is increased. So attractive force of the nucleus for electrons in the outermost shell also increases which in turn gradually decreases the atomic radii.
Q.5 How does the atomic radius change 1050 down a group in the periodic table? Give reason.
Ans. On moving down in any group of the periodic table, the atomic sizes rather the atomic radii of the elements increase.
On moving down a group, a new electronic shell is added to each succeeding elements, though the number of electrons in the outermost shell remains the same. This tends to increase the atomic size.
At the same time, increase in nuclear charge with increase in atomic number tends to decrease the atomic size.
But the effect of addition of new electronic shell is so large that it outweighs the contractive effect of the increased nuclear charge. Hence, there occur a gradual increase in atomic radii on moving down a group in the periodic table.
Q. 6 (1) Arrange in decreasing order of atomic size-O, C, F, Li. (2) Arrange in increasing order of atomic size-Br, F, I, Cl.
Ans. (1) Given elements are members of 2nd period of the periodic table. We know, across a period, atomic radius decreases from left to right. As a result atomic size also decreases in the same fashion. So, the decreasing order of atomic size is Li> C> O> F.
(2) Given elements belong to Gr-17 of the long form of periodic table. We know, down the group atomic radius as well as atomic size gradually increases. So, the increasing order of atomic size is F< Cl< Br< I.
0.7 What is first ionisation energy of an element? Arrange the following elements in the increasing order of their first ionisation energy B, C, N, O and F.
Ans. The minimum amount of energy required to remove the most loosely bound electron from the outermost shell of an isolated gaseous atom of an element in its ground state to form a monopositive ion is called the first ionisation energy of the element.
The ionisation energy of the given elements increases as-B<C<O<N<F.
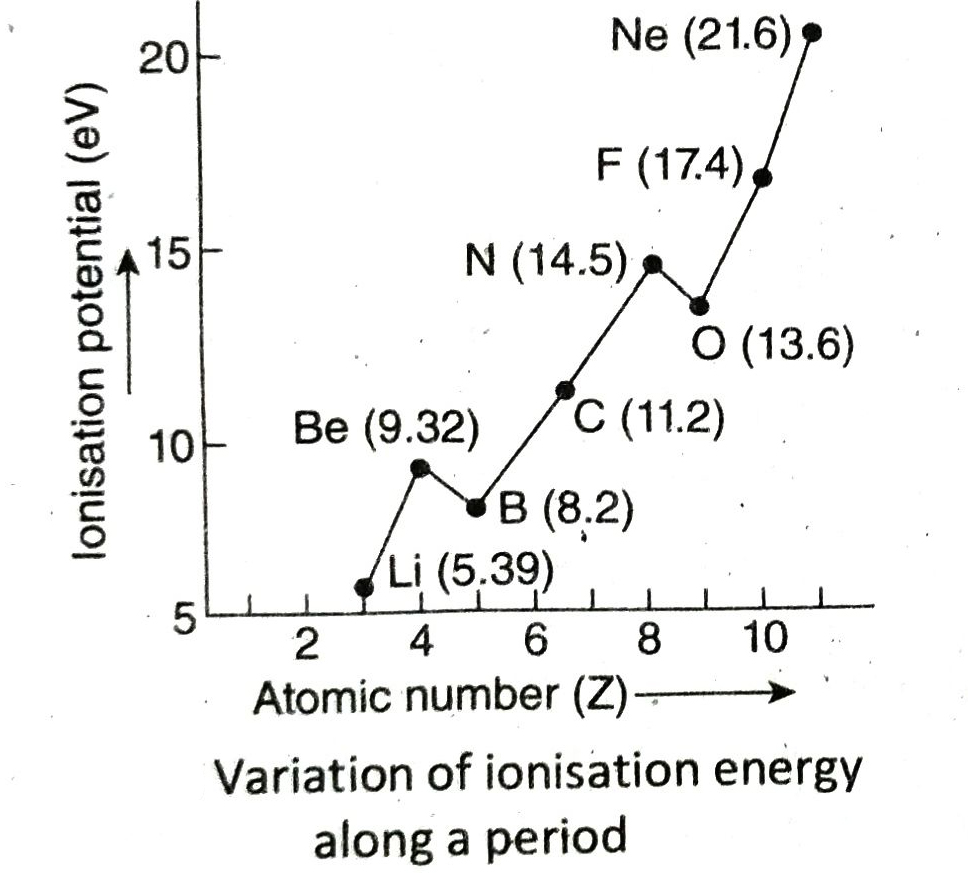
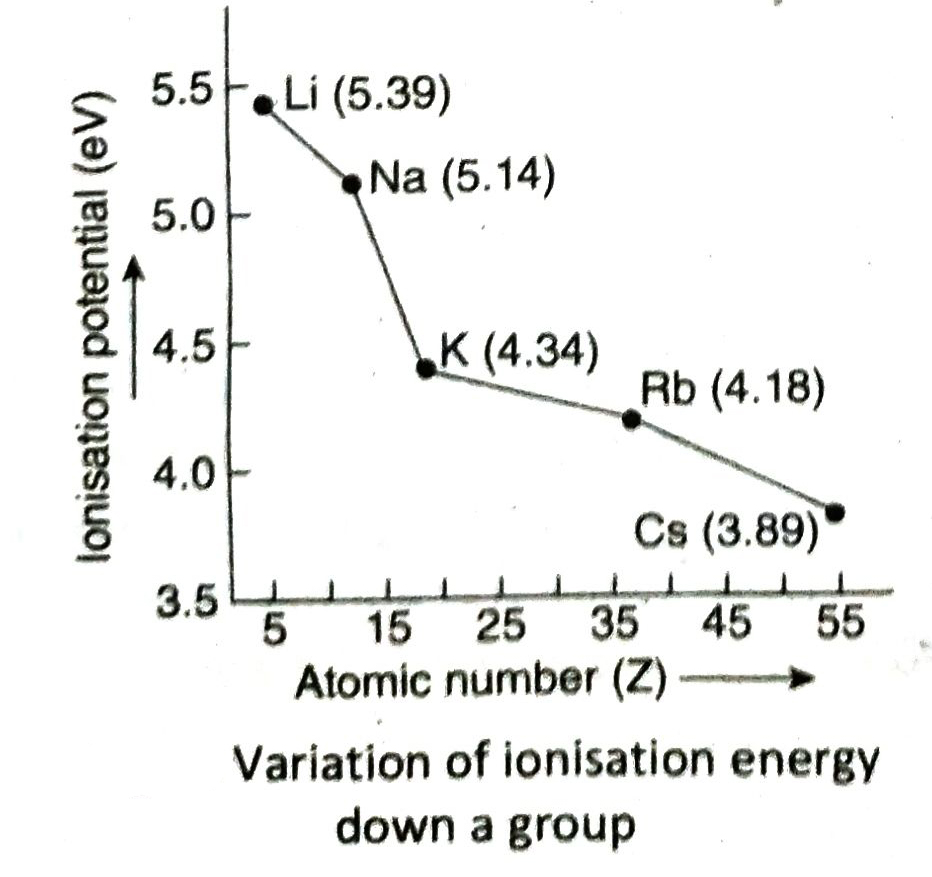
Q.8 How does ionisation energy of elements change along a period and down a group in the periodic table?
Ans. Variation along a period: On moving along a period from left to right, the ionisation energy of the elements generally increases, though some elements show exception to this trend. In a given period, the alkali metals present at the extreme left has the least ionisation energy while the noble gases at the extreme right has the highest ionisation energy.
Variation down a group: The ionisation energy of elements gradually decreases on moving down a group in the periodic table.
Q.9 (1) On which factors does ionisation energy of an element depend? (2) Why ionisation potential of inert elements and alkali metals are very high and very low respectively?
Ans. (1) Factors influencing ionisation energy are-
(a) Atomic size: lonisation energy decreases as the atomic size increases and vice-versa.
(b) Magnitude of nuclear charge: lonisation energy increases with increase in nuclear charge and vice-versa.
(c) Effect of electronic configuration of the outermost shell: lonisation energy increases with increase in stability of the electronic configuration.
(2) Very high value of ionisation energies of inert gases are due to their highly stable electronic configuration. To remove an electron from these highly stable outer shell, higher amount of energy is required making their ionisation energy so high.
On the other hand alkali metals contain only one electron in their valence shell. By the removal of this electron, they can attain the stable electronic configuration of nearest inert gas. This is why removal of electron from alkali metals require lower amount of energy making their ionisation energies low.
Q.10 will ionisation energies of isotopes of an element be equal to each other? Explain.
Ans. Although the number of neutrons in the isotopes of an element are different, number of electrons are same. As a consequence, electronic configurations are also same. That is why ionisation energies of these isotopes becomes equal to each other.
Q.11 Define electronegativity. Name the most electronegative and the least electronegative elements.
Ans. Scientist Pauling defined electronegativity of an element as the tendency of an atom of the element to attract the bonded pair of electrons towards itself when it is covalently bonded to another atom.
The most electronegative element is fluorine (F) and the least electronegative element is caesium (Cs).
Q.12 How does electronegativity of the elements change across a period on moving from left to right? Explain with reasons.
Ans. On moving from left to right across a period the nuclear charge of the elements increases and atomic size decreases. As a result, the nuclear force of attraction on the bonded electron pair increases. Hence, on moving from left to right the electronegativity of elements gradually increases. The alkali metal of a particular period located at the extreme left of the period is the least electronegative while the halogen in group-17 located at the right is the most electronegative element.
Q.13 How does electronegativity of elements change on moving down a group? Arrange the following elements in the increasing order of their electronegativity. F, Br, Cl, I.
Ans. On moving down a group, the atomic size of the elements increases. As a result, the nuclear force of attraction on outermost electrons decreases even though the nuclear charge increases. Hence, on moving down a group electronegativity of the elements gradually decreases.
The increasing order of electronegativity of the given elements: I < Br<CI < F
Q.14 Discuss how the oxidising and reducing power of elements change across a period and down a group in the periodic table.
Ans. Variation across a period: On moving from left to right across a period, the atomic number as well as number of protons gradually increases by one unit. At the same time number of electrons also increases in the same outermost subshell. So, on moving from left to right nuclear attractive force on outermost electrons gradually increases and tendency to lose the electrons decreases. At the same time, tendency to accept electrons increases. As a result, the elements at the left part of a periodic table show a greater tendency to release electrons while the elements at the right part of a periodic table show a greater tendency to accept electrons. Hence, on moving from left to right across a period, the reducing power of the elements decreases whereas oxidising power increases. Alkali metals in group-1 of each period are the strongest reducing agents while the halogens in group-17 of each period are the strongest oxidising agents.
Variation down a group: On moving down a group, atomic size of the elements increases and hence attractive force between the nucleus and the outermost electrons decreases. So, on moving down a group, the tendency of the elements to lose electrons increases. Hence, on moving down a group, reducing power of elements increases whereas oxidising power decreases.
Q.15 Number of electrons and neutrons in an atom of an element are 17 and 18 respectively. Predict the position of the element in the Mendeleev’s periodic table.
Ans. Number of electrons = 17
∴ Electronic configuration = 2, 8, 7
∴ The elements will belong to 3rd period and Group VIII B in Mendeleev’s periodic table.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. Moving across a period from left to right, the atomic radii of elements
A. gradually increases
B. gradually decreases
C. remains unchanged
D. initially increases then decreases
Ans. B
2. On going down a group, the atomic radii of elements
A. gradually decreases
B. gradually increases
C. remains unchanged
D. initially increases then decreases
Ans. B
3. In a given period, with decreasing atomic size of elements, the ionisation energy
A. increases
B. decreases
C. remains unchanged
D. either increases or decreases
Ans. A
4. In a given period, with an increase in nuclear charge of the atom of an element, the ionisation energy
A. decreases
B. increases
C. remains unchanged
D. either increases or decreases
Ans. B
5. The unit of electronegativity is
A. eV
B. erg
C. dyne
D. it is unitless quantity.
Ans. D
6. In a given period, the electronegativity is minimum for the elements of
A. group-1
B. group-2
C. group-14
D. group-17
Ans. A
7. In a given period, the electronegativity is maximum for the elements of group
A. 16
B. 17
C. 18
D. 14
Ans. B
8. In a given period, with increase in atomic size of an element, its electronegativity will
A. increase
B. decrease
C. increases or decrease
D. remain unchanged
Ans. B
9. For the elements of 2nd period, the correct order of atomic radii is
A. B>C>N>O
B. B>N> C>O
C. B>O>N> C
D. N>O > B > C
Ans. A
10. The correct order of atomic radii for alkali metals is
A. Na < Rb < K < Cs
B. Na < K< Rb < Cs
C. K< Na < Cs < Rb
D. Na < Cs <K < Rb
Ans. B
11. The least electronegative non-radioactive element is
A. Na
B. K
C. Rb
D. Cs
Ans. D
12 For the elements of 2nd period, the correct order of ionisation energy is
A. B<C<N>O
B. B<C<O<N
C. C<B<N>O
D. N>C<B<0
Ans. A
13. The correct order of ionisation energy for group-1 elements is
A. Li > K< Rb > Na
B. Li > Na> Rb > K
C. Li > Na > K > Rb
D. Na <Li> Rb > K
Ans. C
14. The correct order of electronegativity for the following element is
A. Li< Be <C<B
B. Li <Be <B<C
C. Li<B> Be > C
D. Be > Li < B<C
Ans. B
15. The correct order of reducing power of the elements in 3rd period is
A. Al < Si > P > S
B. Al > Si > S > P
C. Al> Si > P> S
D. Si < Al > S <P
Ans. C
16. The correct order of oxidising power of the halogens is
A. F> Br > Cl > I
B. Cl < F< Br<1
C. Cl> F >Br > I
D. F> Cl > Br>I
Ans. D
17. Most electronegative element is
A. O
B. Cl
C. F
D. Ne
Ans. C
18. Which one is more electronegative ?
A. Na
B. K
C. Rb
D. Cs
Ans. A
19. Which one of the following has the highest reducing ability ?
A. K
B. Na
C. Li
D. Cs
Ans. D
20. Which halogen element has the least electronegativity?
A. F
B. Cl
C. Br
D. I
Ans. D
21. Which one is not a periodic property?
A. oxidising property
B. reducing property
C. radioactivity
D. Atomic radius
Ans. C
22. Which one of the following has the highest atomic radius?
A. K
B. H
C. Li
D. Na
Ans. A
23. The most oxidising element in second period of the periodic table is
A. F
B. Cl
C. Be
D. O
Ans. A
24. Which of the following alkali metals has the highest atomic radius?
A. Li
B. Na
C. Rb
D. Cs
Ans. D
25. Which of the following indicates the atomic number of a Gr-1A element?
A. 13
B. 17
C. 11
D. 10
Ans. C
Answer in brief
1. What is meant by ‘periodicity of elements’?
Ans. The recurrence of properties of the elements at definite intervals when arranged in increasing order of their atomic numbers is called periodicity of elements.
2. In the case of the 4th period, after how many elements are properties repeated?
Ans. After 18 elements, in the 19th elements properties are repeated.
3. What is meant by the term ‘atomic radius’? Classify atomic radius.
Ans. It is the distance between the centre of the nucleus and the outermost orbit of an atom.
Atomic radius is classified asradius, 2 metallic radius and 3 Waals’ radius. covalent van der
4. Define metallic radius.
Ans. Half of the distance between the nuclei of two adjacent metal atoms in a metallic crystal is called metallic radius.
5. What is van der Waals’ radius?
Ans. Half of the distance between the nuclei of two non-bonded neighbouring atoms of two adjacent molecules of the same substance in solid state is known as van der Waals’ radius.
6. Arrange van der Waals’ radius, metallic radius and covalent radius in decreasing order of their values.
Ans. The decreasing order of the three atomic radii is as follows-
van der Waals’ radius > metallic radius > covalent radius.
7. The elements of which group in a given period of periodic table have the largest atomic radii?
Ans. The elements of group-1 in a given period of the periodic table have the largest atomic radii.
8. Whose atomic radius is smaller-Li or F?
Ans. Atomic radius of F is smaller.
9. Which of the following has the smaller atom – Mg and Al?
Ans. Atoms of Al are smaller than that of Mg.
10. Arrange Be, Na, Ar, S in order of atomic radius.
Ans. Ar > Na > S > Be.
11. Arrange Al, Mg, Cl, Na, P, S and Si in decreasing order of their atomic radii. Decreasing order of atomic radii.
Ans. Na > Mg > Al > Si > P > S >Cl.
12. How does ionisation energy change across a period?
Ans. From left to right, across a period, ionisation energy of elements gradually increases.
13. The elements of which group in a given period of the periodic table have the lowest ionisation energy?
Ans. The elements of group-1 in a given period of the periodic table have the lowest ionisation energy.
14. Name the element with highest ionisation energy.
Ans. Helium (He).
15. Name the element with lowest ionisation energy.
Ans. Caesium.
16. Arrange the following elements in increasing order of ionisation energy-Li, Rb, K and Na.
Ans. Rb<K <Na < Li.
17. What is the relation between atomic radii and electronegativity of the elements in a period?
Ans. Smaller the atomic radii of elements in a period, greater will be the electronegativity.
18. Arrange F, Cl, Br and I in decreasing order of electronegativity.
Ans. Decreasing order of electronegativity: F> Cl > Br>I.
19. How does electronegativity of the Gr-1 elements change from top to bottom?
Ans. From top to bottom of group-electronegativity gradually decreases.
20. Mention the position of the most electronegative element in the periodic table.
Ans. The most electronegative element F belongs to the 2nd period and Gr-17 in the periodic table.
21. Arrange Na, Mg, Al, Cl and P is increasing order of electronegativity.
Ans. Na < Mg < Al < P < Cl.
22. Arrange the following elements in increasing order of electronegativity-Na, O, N, F.
Ans. Na <N<O<F.
23. Arrange Li, Be, B, N in decreasing order of electronegativity.
Ans. N> B > Be > Li.
24. What is the relation between ionisation energy and electronegativity?
Ans. The ionisation energy of an element increases with the increase in electronegativity.
25. What is the relation between ionisation energy and reducing property of an element?
Ans. Lower the ionisation energy of an element, stronger will be its reducing power.
26. What is the relation between electronegativity and oxidising property of an element?
Ans. Higher the electronegativity of an element, stronger will be its oxidising power.
27. An element A is positioned in group-13 of periodic table. What is the formula of its chloride?
Ans. The valency of all elements of group-13 is 3. Hence, the formula of the chloride of the element A will be ACl3.
28. Which elements among the halogens exhibit highest and lowest metallic property?
Ans. Among the halogens, iodine exhibits highest metallic property while fluorine exhibits lowest metallic property.
29 Arrange Si, Mg, P, Al, Na, S and Cl in increasing order of their oxidising power.
Ans. Increasing order of oxidising power: Na <Mg < Al < Si < P<S<Cl.
30. Elements of which group in the periodic table has the highest oxidising power?
Ans. Elements of group-17 of the periodic table have the highest oxidising power among the elements of the corresponding period.
31. Name the strongest oxidising agent of 2nd period.
Ans. Fluorine (F) is the strongest oxidising agent of 2nd period.
32. Name the element with highest reducing ability of Gr-1 in the long form of periodic table.
Ans. Lithium (Li).
33. Among Na, K, O and N which one is the strongest oxidising agent and strongest reducing agent?
Ans. Strongest oxidising agent is O and strongest reducing agent is K.
34. Give an example of a halogen that has reducing property.
Ans. lodine.
Fill in the blanks
1. ………… is not a periodic property of elements.
Ans. Radioactivity
2. In a given period the ………… have the smallest atomic radii.
Ans. halogens
3. The atomic radii of noble gases in a given period is ………. than the atomic radii of halogen atoms.
Ans. greater
4. The minimum energy required to remove the most loosely bound electron in the outermost orbit of the atom of an element is known as …………. energy of the element.
Ans. ionisation
5. The electronegativity of elements is measured in ………… scale.
Ans. Pauling
6. The element ………… is the strongest oxidising agent.
Ans. fluorine
7. Higher the ………… of an element, stronger will be its oxidising power.
Ans. electronegativity
8. Noble gases being ……….. their covalent radii cannot be measured.
Ans. mono-atomic
9. Greater the tendency of an element to accept electron, greater will be its ………. property and greater the tendency of an element to release electron, greater will be its ……….. property.
Ans. oxidising, reducing
10. The atomic radius of Al is ………… than Si.
Ans. greater
11. The ionisation potential of oxygen is ……….. than that of nitrogen.
Ans. less
12. The ………… power of Na is less than that of Si.
Ans. oxidising
13. The reducing power of caesium is ………… than that of rubidium.
Ans. more
14. Magnetic property of elements is not a ……….. property.
Ans. periodic
15. Across a period from left to right atomic radius of elements gradually …………
Ans. decreases
16. Among all elements, atomic radius is the least in case of …………..
Ans. hydrogen
17. Among Al, S, Si and P, element with highest electronegativity is ………..
Ans. S
18. The most reactive non-metal is ………….
Ans. fluorine
State whether true or false
1. Covalent radius is always greater than metallic radius.
Ans. False
2. The correct order of atomic size is – F< Cl< Br < I< At
Ans. False
3. Greater the size of an atom of an element, greater will be its electronegativity.
Ans. False
4. In the measurement of electronegativity of different elements elements by Pauling scale, electronegativity of hydrogen is taken as 2.1.
Ans. True
5. The increasing order of electropositivity is – Na <K < Rb < Cs
Ans. True
6. Mendeleev emphasized mainly on chemical and physical properties of elements while constructing the periodic table.
Ans. True
7. Down a group atomic size of an element gradually decreases.
Ans. False
8. lonisation energy decreases with increase in atomic size.
Ans. True
9. Down a group metallic character decreases and oxidising power increases.
Ans. False
10. Across a period, from left to right oxidising property decreases.
Ans. False
11. Halogen elements are strong reducing agent.
Ans. False
12. Order of reducing power-F> Cl > Br>I.
Ans. False
13. If the number of electrons in the valence shell is 3, then the position of element in the long form of periodic table will be in group 13.
Ans. True