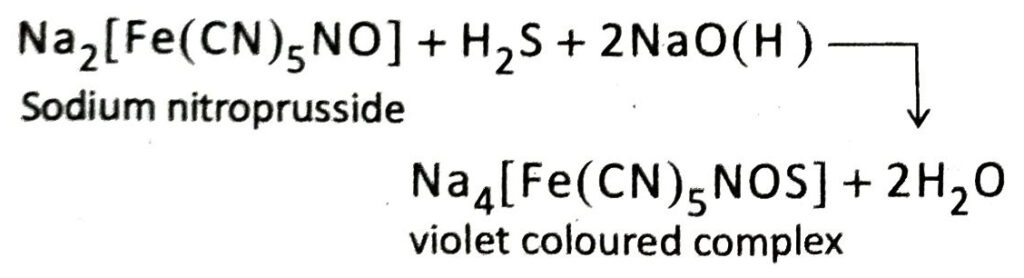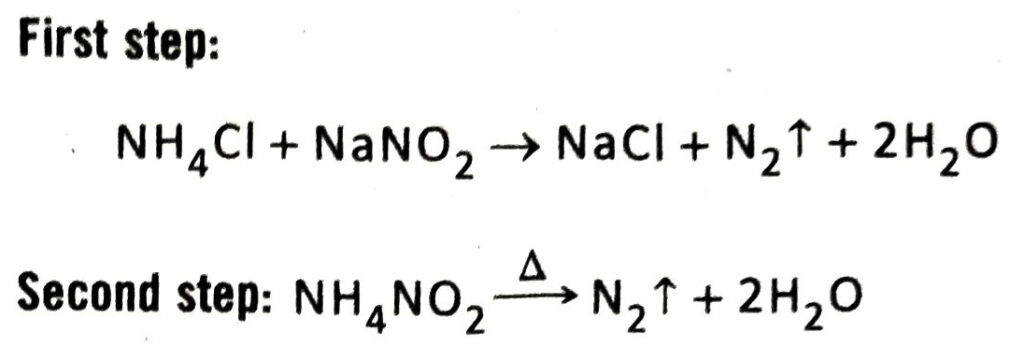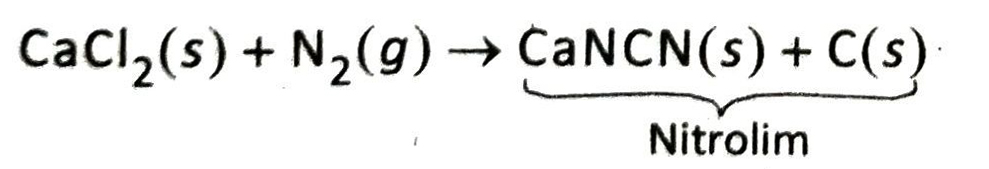WBBSE 10th Class Science Solutions Physical Science & Environment Chapter 8.4 Inorganic Chemistry in Laboratory and Chemical Industry
West Bengal Board 10th Class Science Solutions Physical Science & Environment Chapter 8.4 Inorganic Chemistry in Laboratory and Chemical Industry
WBBSE 10th Class Physical Science & Environment Solutions
8.4 Inorganic Chemistry in Laboratory and Chemical Industry
Synopsis
Ammonia and Urea
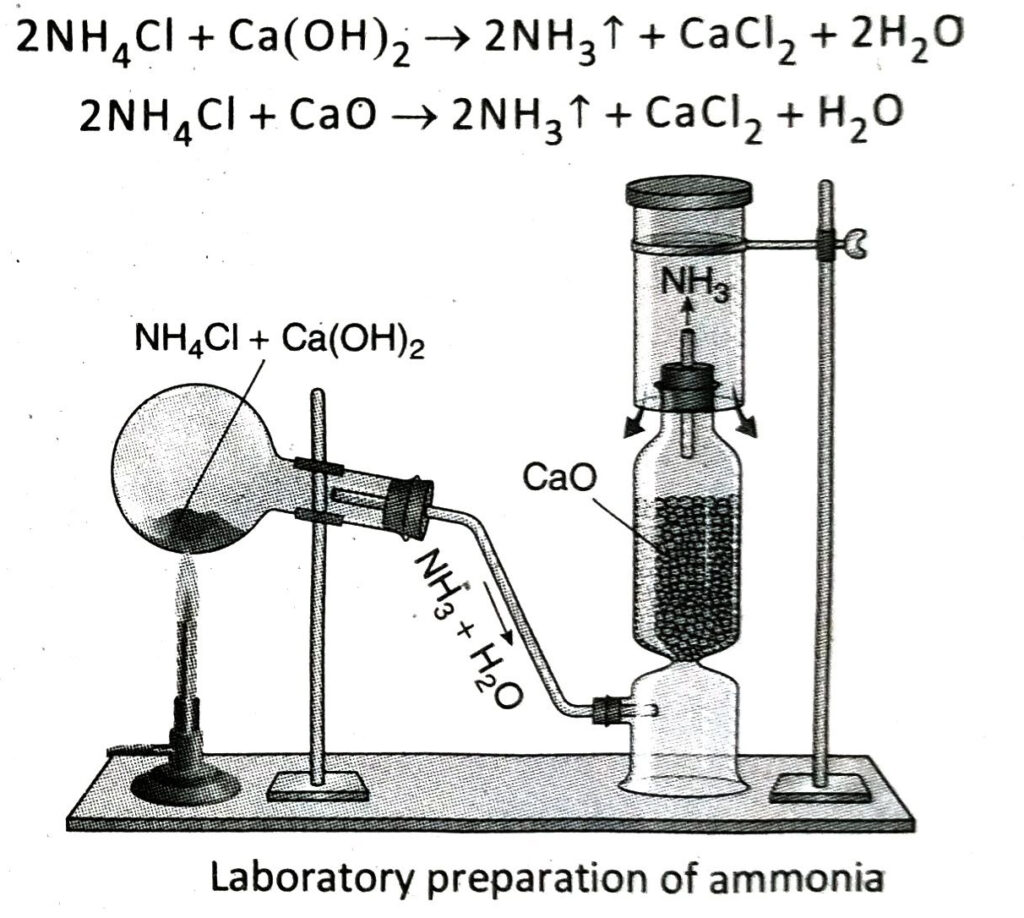
- Laboratory preparation of NH3 : Ammonia (NH3) is prepared in the laboratory by heating a mixture of ammonium chloride and anhydrous slaked lime taken in a ratio of 1 : 3 by mass.
- Drying and collection of NH3 : Water vapour is then removed from the ammonia gas by passing the gas through a column of quick. lime and then it is collected by downward displacement of air.
- Physical properties of NH3 : Ammonia is a colourless and pungent smelling gas. It is highly soluble in water. A saturated aqueous solution of ammonia having a specific gravity of 0.88 is known as liquor ammonia.
- Chemical properties of NH3 : (1) The aqueous solution of ammonia is alkaline in nature. (2) Ammonia exhibits strong reducing property and can precipitate metal ions such as Fe3+ , Al3+ etc., as insoluble hydroxides from their aqueous solution. (3) Nessler’s reagent turns brown in presence of ammonia. (4) When excess NH3 gas is passed through CuSO4 solution, the solution turns deep blue.
- Industrial preparation of ammonia by Haber’s process: Ammonia is industrially prepared by Haber’s process by the direct combination of 1 volume of N2 gas and 3 volumes of H2 gas. The reaction is carried out at an optimum temperature and a pressure of 450°C and 200 atm respectively. Iron oxide is used as the catalyst and a mixture of K2O and Al2O3 is used as the promoter in this reaction.
- Preparation of urea: Urea [CO(NH2)2] is commercially manufactured by heating a mixture of liquid NH3 and CO2 gas in a closed vessel at 170-190°C temperature and a pressure of about 175 atm.
Reactions:
- Uses of ammonia: (1) NH3 gas and aqueous solution of ammonia are extensively used in the laboratories as important reagents. (2) Liquid ammonia is used as a refrigerant in refrigerators, cold storages and ice manufacturing units. (3) Ammonia is also used to prepare urea.
- Uses of urea: (1) Urea is widely used as nitrogenous fertiliser. (2) It is also used in the preparation of different medicines such as urea stibamine, barbiturates etc. (3) It is also used in the commercial preparation of nitrocellulose, cellophane etc.
TOPIC – A
Ammonia and Urea
SHORT AND LONG ANSWER TYPE QUESTIONS
Q.1 Why is concentrated H2SO4 or phosphorus pentoxide (P2O5) or anhydrous CaCl2 not used to dry NH3 gas prepared in the laboratory?
Ans. Ammonia being a basic compound cannot be dried by using concentrated H2SO4 or P2O5 as these are acidic in nature. This is because concentrated H2SO4 and P2O5 react with ammonia to produce ammonium sulphate and ammonium phosphate respectively.
2NH3 + H2SO4 → (NH4)2SO4 ;
6NH3 + P2O5 + 3H2O → 2(NH4)3PO4
On the other hand, anhydrous CaCl2 absorbs ammonia to form an addition compound. Hence, it is also not suitable to dry NH3 gas prepared in the laboratory.
CaCl2 + 8NH3 → CaCl2 · 8NH3
(addition compound)
Q.2 What precautionary measures should be taken if an accident occurs due to leakage of ammonia gas in cold storages, factories or from storage tanks containing ammonia?
Ans. Ammonia is a basic gas with a characteristic strong, pungent smell. It is extremely harmful to eyes and can cause respiratory troubles if it is inhaled.
Large quantities of ammonia can dissolve in a small volume of water. So, in case of ammonia leakage, the eyes and face of the affected person should be immediately washed with plenty of water and the face of the person should be covered with a wet cloth or handkerchief to avoid further contact with ammonia. Then the person should be immediately taken to the doctor. In addition to this, plenty of water should be sprayed at the site of the leakage so that ammonia gas present in the air is absorbed by water, thereby diminishing the chance of further harmful effects.
Q.3 State the principle and suitable chemical equation for the industrial preparation of urea.
Ans. Urea is industrially prepared by heating a mixture of liquid ammonia and carbon dioxide gas in a closed vessel at around 170-190°C and under 175 atmospheric pressure.
Chemical equation: The reaction takes place in two steps-
- In the first step, liquid ammonia and carbon dioxide gas react with each other to form ammonium carbamate (H2NCOONH4). This step is reversible in nature.
- In the second step, ammonium carbamate decomposes to form urea (H2NCONH2). This step is also reversible in nature.
The first step almost proceeds towards completion, but in the second step only 40-60% of ammonium carbamate gets converted into urea.
Q.4 Mention some important uses of ammonia in different industries.
Ans. Important uses of ammonia are-
- Ammonia is used to manufacture important nitrogenous fertilisers such as ammonium sulphate, ammonium nitrate, ammonium phosphate, urea etc.
- It is used to produce nitric acid by Ostwald’s process and sodium carbonate by Solvay process.
- Liquid ammonia is used as a refrigerant in cold storages, ice factories, refrigerators etc.
Q.5 State some important uses of urea.
Ans. Some important uses of urea are-
- It is extensively used as a nitrogenous fertiliser for agricultural purposes.
- Urea stibamine, a medicine for treating of Kala azar, is prepared from urea. Barbiturates (a kind of sedative) is also made from urea.
- Urea is used as a raw material in the industrial preparation of cellophane papers, rayons, nitrocellulose (an explosive) etc. It is used to make urea-formaldehyde resin.
Q.6 State the principle and chemical reactions involved in the laboratory preparation of ammonia.
Ans. Principle: In the laboratory, ammonia is prepared by heating a mixture of ammonium chloride (NH4Cl) and slaked lime [Ca(OH)2] or quick lime (CaO).
Chemical reactions:
Q.7 How is water vapour removed from ammonia gas produced in the laboratory? Why is a particular drying agent used in this case?
Ans. Ammonia gas produced in the laboratory is passed through a column containing quick lime (CaO) with the help of a delivery tube. Quick lime absorbs water vapour present in ammonia gas and as a result, dry ammonia gas is obtained.
NH3, being a basic compound, cannot be dried up using acidic compounds. Hence a particular drying agent should be used to dry ammonia. Therefore a basic substance, such as, quick lime (CaO) is used for drying ammonia in the laboratory.
Q.8 In the laboratory preparation of ammonia, NH4Cl and Ca(OH)2 are mixed thoroughly in their dry states and then heated. Why?
Ans. Dry NH4Cl undergoes sublimation on heating, i.e., it directly vapourises from its solid state. So, if NH4Cl and Ca(OH)2 are not mixed thoroughly, NH4Cl on heating will sublime and get eliminated from the reaction mixture. The mixture of NH4Cl and Ca(OH)2 is heated in their dry states because ammonia is highly soluble in water and thus, on heating its aqueous solution, ammonia gas cannot be obtained in its free state.
Q.9 How will you prepare ammonia gas in the laboratory at room temperature?
Ans. When liquor ammonia is added dropwise on solid caustic potash or caustic soda, water gets absorbed by the alkali thereby liberating ammonia gas.
Q.10 Why is liquid ammonia used as refrigerant?
Ans. The latent heat of vaporisation of liquid ammonia is very high. So, it absorbs large amount of heat from the surroundings during evaporation. This physical property of liquid ammonia makes it very useful as a refrigerant.
Q.11 We get a strong pungent smell of ammonia near laboratories and stables. Why?
Ans. The urine of humans and other mammals contains urea (NH2CONH2) which is decomposed by bacteria to produce ammonia. This is why we get a strong pungent smell of ammonia near laboratories and stables.
Q.12 is it justified to term the aqueous solution of ammonia as ammonium hydroxide?
Ans. There is no existence of NH4OH molecules in the aqueous solution of ammonía. So, an aqueous solution of ammonia cannot be termed as ammonium hydroxide rather, it is justified to term it as aqueous ammonia.
Q.13 Write with equation what happens when ammonia gas comes in contact with hydrogen chloride gas.
Ans. When ammonia gas comes in contact with hydrogen chloride gas, dense white fumes are formed. These fumes are actually the aggregation of fine ammonium chloride (NH4Cl) particles floating in air.
NH3 (g) + HCl(g) → NH4Cl(s)
Q.14 Write with equation what happens when chlorine gas reacts with excess ammonia.
Ans. When chlorine gas reacts with excess ammonia, the latter gets oxidised to nitrogen while the former gets reduced to ammonium chloride.
8NH3 + 3Cl2 → N2 ↑ + 6NH4Cl
Q.15 Give an example of the reducing property of ammonia.
Ans. When ammonia is passed over heated cupric oxide, the latter is reduced to form red metallic copper and ammonia itself gets oxidised to nitrogen.
Q.16 Write with equation what happens when ammonia gas is passed over heated lead oxide.
Ans. When ammonia is passed over heated lead oxide, yellow coloured lead oxide is reduced to form a grey precipitate of metallic lead while ammonia itself gets oxidised to nitrogen.
Q.17 Write with equation what happens when an aqueous solution of ammonia is added to an aqueous solution of ferric chloride.
Ans. When an aqueous solution of ammonia is added to a yellow coloured solution of ferric chloride, a brown precipitate of ferric hydroxide is produced.
Q.18 What happens when aqueous solution of ammonia is added to an aqueous solution of aluminium chloride? Give equation.
Ans. When aqueous solution of ammonia is added to a colourless aqueous solution of aluminium chloride, a white gelatinous precipitate of aluminium hydroxide is produced.
Q.19 What happens when ammonia reacts with excess chlorine gas? Give equation.
Ans. When ammonia reacts with excess chlorine gas, a yellow coloured oily explosive compound, nitrogen trichloride (NC13) is formed along with hydrogen chloride.
Reaction: NH3 + 3Cl2 → NCl3 + 3HCl
Q.20 What happens when ammonia is oxidised in presence of a catalyst? Give equation.
Ans. When a mixture of ammonia and excess oxygen is passed over heated platinum or a wire-gauze made of platinum-rhodium alloy (catalyst) at around 700°C, ammonia is oxidised to produce nitric oxide.
Reaction: 4NH3 + 5O2 → 4NO + 6H2O
Q.21 What is meant by ‘liquor ammonia’ and ‘liquor ammonia’ and liquid ammonia’?
Ans. Liquor ammonia: A concentrated aqueous solution of ammonia containing 35% ammonia is known as ‘liquor ammonia’. It has a specific gravity of almost 0.88.
Liquid ammonia: Ammonia is condensed to a colourless liquid at around -33.4°C temperature and at normal atmospheric pressure. This is known as ‘liquid ammonia’.
Q.22 Why is a bottle containing liquor ammonia cooled before opening?
Ans. In a bottle of liquor ammonia, ammonia gas is dissolved in water under high pressure. So, when the bottle is opened, the ammonia gas dissolved in water immediately comes out of the bottle with great force. This may cause serious eye injuries. On the other hand, if the bottle is cooled, then the solubility of ammonia in water increases as a result of which the pressure inside the bottle decreases. So on opening the bottle, ammonia does not spout.
Q.23 What changes are observed when an aqueous solution of ammonia is added to an aqueous solution of copper sulphate?
Ans. When an aqueous solution of ammonia is added to a blue coloured aqueous solution of copper sulphate, initially a bluish-white precipitate is formed. On addition of excess ammonia solution, the precipitate dissolves and the solution turns deep blue.
Q.24 What is Nessler’s reagent? What happens when ammonia comes in contact with Nessler’s reagent?
Ans. An alkaline solution of potassium mercuric iodide [K2Hgl4] is known as Nessler’s reagent.
When ammonia comes in contact with a small amount of Nessler’s reagent it forms a brown solution. When excess ammonia is passed through Nessler’s reagent, a brown precipitate is formed.
Q.25 State the principle of preparation of ammonia by Haber’s process along with suitable chemical equation.
Ans. When a mixture of nitrogen and hydrogen (in the volumetric ratio of 1:3) is heated at a temperature of about 450°C and 200 atmospheric pressure in the presence of iron oxide as catalyst and a mixture of K2O and Al2O3 as promoter, ammonia gas is produced by the direct combination of nitrogen and hydrogen.
Chemical equation:
Q. 26 How is the dry ammonia gas collected at laboratory? Why is ammonia gas not collected by downward displacement of water?
Ans. Ammonia gas is lighter than air. Hence, at laboratory, the dry ammonia gas is collected by the downward displacement of air in an inverted dry gas jar.
Ammonia gas is highly soluble in water. Hence, if it is collected by downward displacement of water it promptly gets dissolved in the water and forms ammonium hydroxide. Moreover to fill the vacuum caused by the dissolution of ammonia, water enters the hot round bottom flask. As a consequence the flask bursts. That is why ammonia is not collected by downward displacement of water.
Q.27 Why aqueous solution of ammonia can not be concentrated by heating?
Ans. On applying heat to the aqueous solution of ammonia, the dissolved ammonia gets evaporated from the solution. Hence amount of ammonia in the solution gets reduced and the solution becomes diluted instead of getting concentrated.
Q.28 What is vanishing ink? Why is it named so?
Ans. The aqueous solution of ammonia mixed with a few drops of phenolphthalein indicator is called vanishing ink. The solution is pink in colour..
The aqueous solution of ammonia is alkaline in nature. On addition of a few drops of phenolphthalein the solution turns pink. Since ammonia is volatile in nature it evaporates from the solution. As a result the solution becomes neutral is nature. As phenolphthalein is colourless in neutral medium, the pink colour of the ammonia solution vanishes after some time. Hence it is called ‘vanishing ink’.
Q. 29 Prove that ammonia contains hydrogen.
Ans. When ammonia gas is passed over heated (360°C) sodium metal, a colourless odourless gas is formed. If a lighted splint is exposed to the gas, it starts burning with a squeaky pop and a blue flame but the flaming splint gets extinguished. Hence the gas is hydrogen and this hydrogen comes from ammonia. Hence it can be concluded that ammonia contains hydrogen.
2Na+ 2NH3 → 2NaNH2 + H2
Q.30 Prove that ammonia contains nitrogen.
Ans. When ammonia gas is passed over heated cupric oxide, a colourless and odourless gas is obtained. Now if a burning Mg-ribbon is introduced to the gas, it continues burning and a white residue is produced. The white residue, such obtained, when boiled with water, a pungent smelling gas evolves which turns the alkaline Nessler’s reagent brown. Therefore, this pungent smelling gas is ammonia. Hence the white residue is of magnesium nitride and the colourless, odourless gas is nitrogen. So it can be concluded that ammonia contains nitrogen.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. Which of the following substances is present in the urine of animals?
A. NH2Cl
B. (NH4)2SO4
C. CO(NH2)2
D. Ca(OH)2
Ans. C
2. Which of the following does not react with water to form ammonia?
A. Mg3N2
B. NO2
C. AIN
D. CaNCN
Ans. B
3. Ammonia is dried by using
A. P2O5
B. anhydrous CaCl2
C. concentrated H2SO4
D. quick lime (CaO)
Ans. D
4. The nature of aqueous solution of ammonia
A. is acidic
B. is alkaline
C. is neutral
D. changes according to conditions
Ans. B
5. If excess ammonium hydroxide is added to copper sulphate solution, then the solution turns
A. deep red
B. deep yellow
C. deep violet
D. deep blue
Ans. D
6. In the presence of Nessler’s reagent, ammonia turns
A. black
B. green
C. brown
D. yellow
Ans. C
7. The catalyst used in the catalytic oxidation of ammonia to produce NO is
A. MnO2
B. platinum
C. iron
D. copper
Ans. B
8. The amount of ammonia present in liquor ammonia is
A. 25%
B. 30%
C. 35%
D. 45%
Ans. C
9. The industrial preparation of ammonia is carried out by
A. Ostwald’s process
B. Solvay process
C. Bayer’s process
D. Haber’s process
Ans. D
10. The catalyst used in the preparation of ammonia by Haber’s process is
A. MnO2
B. platinum
C. iron oxide
D. copper
Ans. C
11. The reaction involved in the production of ammonia by Haber’s process is a type of
A. isochoric reaction
B. isobaric reaction
C. endothermic reaction
D. exothermic reaction
Ans. D
12. Which of the following is used as a promoter in Haber’s process of ammonia synthesis?
A. K2O , Al2O3
B. Na2O , Al2(SO4)3
C. Na2O , Al2O3
D. K2O , Al2(SO4)3
Ans. A
13. The volumetric ratio in which N2 and H2 are mixed in Haber’s process is
A. 2:3
B. 3:1
C. 1:3
D. 3:2
Ans. C
14. The compound formed in the first step of industrial preparation of urea is
A. ammonium carbamate
B. ammonium carbonate
C. ammonium sulphate
D. ammonium sulfite
Ans. A
15 The aqueous solution of which of the following salts does not form any precipitate when NH3 gas or NH4OH solution is passed through it?
A. CuSO4
B. AlCl3
C. FeCl3
D. NH4NO3
Ans. D
16. Ammonia does not react with
A. NaOH
B. H2SO4
C. HCl
D. H3PO4
Ans. A
17. Ammonia gas is collected at laboratory by
A. upward displacement of air
B. upward displacement of water
C. downward displacement of air
D. downward displacement of water
Ans. C
18. The optimum temperature for the industrial preparation of ammonia in Haber’s process
A. 723°C
B. 723K
C. 813K
D. 823K
Ans. B
19. On cooling ammonia gas converts to a colourless liquid at
A. 0°C
B. 25°C
C. –33.4°C
D. 30°C
Ans. C
20. Which of the following metallic salt solutions reacts with aqueous solution of NH3 to form a brown precipitate?
A. Al3+ salt
B. Cu2+ salt
C. Fe3+ salt
D. Ni2+ salt
Ans. C
21. Which of the following gases can be identified by Nessler’s reagent?
A. NH3
B. H2S
C. N2
D. CO2
Ans. A
22. Which of the following is obtained by heating ammonium carbamate?
A. NH3
B. CO(NH2)2
C. CO2
D. N2
Ans. B
23. Which of the following is obtained by heating ammonium nitrate and calcium oxide?
A. N2
B. NO2
C. NH3
D. NO
Ans. C
24. Which of the following forms a brown precipitate with ferric chloride solution?
A. NH4Cl
B. NH4OH
C. NaCl
D. CuSO4
Ans. B
25. Substance used in the preparation of medicine of human leishmaniasis is
A. ammonia
B. hydrogen sulphide
C. urea
D. sulphuric acid
Ans. C
26. Solubility of which gas in water is the highest?
A. N2
B. NH3
C. H2S
D. CO2
Ans. B
27. Which of the following is used in ice factories for cooling purpose?
A. NH3
B. CO2
C. NH2OH
D. CHCl3
Ans. A
28. The gas formed by passing NH3 gas over heated CuO is
A. H2
B. O2
C. H2S
D. N2
Ans. D
29. Which of the following creates white fumes with HCl?
A. CO2
B. H2S
C. N2
D. NH3
Ans. D
30. Formula of Nessler’s reagent is
A. K2Hgl4 + KOH
B. K2SO4 · 10H2O + KOH
C. K2HgCl4 + KOH
D. K2Mgl4 + KOH
Ans. A
Answer in brief
1. Name the chemical substances required for the laboratory preparation of ammonia.
Ans. Ammonium chloride (NH4Cl) and dry slaked lime [Ca(OH)2] are used for the laboratory preparation of ammonia.
2. What happens when moist ammonia gas is treated with phosphorus pentoxide?
Ans. Moist ammonia gas reacts with phosphorus pentoxide (P2O5) to form ammonium phosphate [(NH4)3PO4] salt.
3. What is the specific gravity of liquor ammonia?
Ans. Specific gravity of liquor ammonia is 0.88.
4. Write down the formula of the addition compound formed when ammonia is absorbed by anhydrous CaCl2.
Ans. The formula of the addition compound is CaCl2 · 8NH3
5. Write the name of the oily explosive liquid that is produced by the reaction of excess chlorine with ammonia.
Ans. The oily explosive liquid is nitrogen trichloride (NCl3).
6. What can be concluded from the fountain experiment?
Ans. The fountain experiment proves that ammonia is highly soluble in water and its aqueous solution is alkaline in nature.
7. Give an example of a reaction where two gases react to form a solid.
Ans. When ammonia gas reacts with hydrogen chloride gas, dense white fumes of solid ammonium chloride (NH4CI) are formed.
NH3(g) + HCl(g) → NH4Cl(s)
8. Which gas is produced by the combustion of ammonia gas in the presence of oxygen?
Ans. Nitrogen (N2) gas is produced.
9. What is the colour of the precipitate formed when ammonia gas is passed through aqueous solution of ferric salts?
Ans. Reddish brown precipitate of ferric hydroxide [Fe(OH)3] is formed in this case.
FeCl3 + 3NH4OH → Fe(OH)3 ↓ + 3NH2Cl
10. What is the colour of the precipitate formed when ammonia gas is passed through aqueous solution of aluminium salts?
Ans. In this case, white gelatinous precipitate of aluminium hydroxide [Al(OH)3] is formed.
AlCl3 + 3NH4OH → Al(OH)3 ↓ + 3NH4Cl
11. Why should a bottle of liquor ammonia always be cooled before opening?
Ans. On cooling, the solubility of ammonia in water increases which prevents accidental spouting of NH3 gas from the bottle.
12. Name an inorganic fertilizer which is prepared from ammonia.
Ans. An inorganic fertiliser which is prepared from ammonia is ammonium phosphate [(NH4)3PO4].
13. Name the chemical compounds that are used as raw materials for the industrial preparation of urea.
Ans. Liquid ammonia and carbon dioxide are used as raw materials for the industrial preparation of urea.
14. What is the percentage of nitrogen present in urea?
Ans. The amount of nitrogen present in urea is almost 46% by mass.
15. Which nitrogenous organic compound is used to prepare medicine for kala azar or black fever?
Ans. Urea is used to prepare medicine for kala azar or black fever.
16. Why is liquid ammonia used as a coolant?
Ans. Liquid ammonia is used as a coolant due to its high latent heat of vaporisation.
17. State whether the chemical reaction involved in Haber’s process of ammonia synthesis is exothermic or endothermic.
Ans. The chemical reaction involved in Haber’s process of ammonia synthesis is exothermic.
18. Name a promoter which can be used instead of molybdenum (Mo) dust in Haber’s process of ammonia synthesis.
Ans. A mixture of Al2O3 and K2O can be used as a promoter instead of Mo dust in Haber’s process of ammonía synthesis.
19. Which nitrogenous compound is produced by the hydrolysis of calcium cyanamide?
Ans. Ammonia (NH3) is produced by the hydrolysis of calcium cyanamide.
20. Which gas when passed over heated CuO produces N2 gas?
Ans. Ammonia gas when passed over heated CuO produces N2 gas.
21. By the decomposition of which element, present in the urine of vertebrates, ammonia gas is released?
Ans. Urea,
22. Which gas is termed as alkaline air?
Ans. Ammonia gas is termed as alkaline air.
23. How is ammonia gas collected at laboratory?
Ans. Ammonia gas is collected at laboratory by the downward displacement of air.
24. Name the process by which ammonia gas is prepared commercially.
Ans. Haber’s process.
25. Name a gas which turns the moist red litmus paper blue?
Ans. Ammonia (NH3).
26. Name a gas which forms dense white fumes when it comes in contact to HCl?
Ans. Ammonia (NH3).
27. Which gas is formed by the reaction of ammonia with sodium?
Ans. Hydrogen (H2 )gas is formed by the reaction of ammonia with sodium.
28. Which gas is identified using Nessler’s reagent?
Ans. Ammonia gas.
29. Mention an use of liquid ammonia.
Ans. Liquid ammonia is used as a refrigerant in cold storages or in ice factories.
30. What is liquor ammonia?
Ans. Concentrated aqueous solution of ammonia (specific gravity = 0.88) is termed as liquor ammonia. In liquor ammonia almost 35% ammonia is dissolved in water under high pressure.
31. Write down the name and formula of a nitrogenous organic fertiliser.
Ans. Urea (formula H2NCONH2).
32. Write down the conditions for the preparation of urea from ammonia and carbon dioxide.
Ans. Urea is prepared by the reaction of liquid ammonia and carbon dioxide at around 175 atmospheric pressure and at a temperature of 170-190°C.
33. Mention an use of urea.
Ans. Urea is used as a nitrogenous organic fertiliser.
Fill in the blanks
1. Ammonia gas is ………. than air.
Ans. lighter
2. Anhydrous CaCl2 absorbs ammonia to form an ………. compound.
Ans. addition
3. Quicklime can be used to dry ammonia gas because it is …………. in nature.
Ans. basic
4. At normal temperature and pressure ………… mL ammonia gas can dissolve in 1 mL water.
Ans. 1300
5. The reaction between FeCl3 and NH4OH is a ………… reaction.
Ans. double displacement
6. Concentrated H2SO4 absorbs ammonia to form ………… salt.
Ans. (NH4)2SO4
7. …………. is widely used as a non-aqueous solvent.
Ans. Liquid ammonia
8. The chemical name of urea is …………..
Ans. carbamide
9. An example of a nitrogenous organic fertiliser is …………
Ans. urea
10. Ammonia can not be dried up using concentrated H2SO4 as ammonia is an ……….. substance.
Ans. alkaline
11. Optimum temperature and pressure for the preparation of ammonia by Haber’s process ……….. is ……….. and ……….. respectively.
Ans. 450°C, 200atm
12. Vapour density of ammonia gas is …………
Ans. 8.5
13. The latent heat of vaporisation of liquid ammonia is very …………
Ans. high
14. The deep blue complex salt formed by dissolving the compound formed by adding ammonia solution in CuSO4 solution in excess NH4OH is …………
Ans. Cu(NH3)4SO4
State whether true or false
1. In the laboratory preparation of ammonia, ammonium chloride and calcium hydroxide are taken in a ratio of 3:1 by mass.
Ans. False
2. Liquor ammonia contains about 50% of ammonia.
Ans. False
3. The addition compound formed due to the reaction between anhydrous calcium chloride and ammonia is CaCl2 · 10NH3.
Ans. False
4. Trace amount of ammonia present in air or water can be detected by its reaction with Nessler’s reagent.
Ans. True
5. The chemical reaction involved in preparation of ammonia by Haber’s process is an endothermic reaction.
Ans. False
6. Urea is used to prepare barbiturates which is a kind of tranquilizer.
Ans. True
7. Ammonia was initially named as ‘alkaline air’ by Priestly.
Ans. True
8. Nessler’s solution turns brown by absorbing ammonia gas.
Ans. True
9. Liquor ammonia is aqueous solution of ammonia.
Ans. True
10. In case of ammonia leakage, you have to give a splash of water in the eyes and all over your face.
Ans. True
11. The required pressure in Haber’s process is 2 atm.
Ans. False
12. Iron dust is used as catalyst in the industrial preparation of ammonia from N2 and H2.
Ans. True
13. Urea is used to prepare cellophane and rayon.
Ans. True
14. Catalytic oxidation of ammonia produces nitric oxide.
Ans. True
TOPIC – B
Hydrogen sulphide and Nitrogen
SHORT AND LONG ANSWER TYPE QUESTIONS
Q.1 Why is concentrated H2SO4 or anhydrous CaCl2 or quick lime (CaO) not used to dry H2S gas prepared in the gas prepared in laboratory?
Ans. If concentrated H2SO4 is used in drying H2S gas, then H2S is oxidised by concentrated H2SO4 to sulphur. So, concentrated H2SO4 is not suitable for drying H2S gas prepared in the laboratory.
H2S + H2SO4 → S↓ + SO2 ↑ + 2H2O
On the other hand, CaCl2 reacts with H2S to form calcium sulphide and liberates hydrogen chloride gas. So, anhydrous CaCl2 is not a suitable drying agent for H2S.
CaCl2 + H2S → CaS + 2HCl
H2S being an acidic compound reacts with bases such as quick lime to produce calcium sulphide and water. Thus, quick lime cannot be used to dry H2S gas prepared in the laboratory.
CaO + H2S → CaS + H2O
Q.2 Why does the white colour of objects made of silver or oil paintings slowly turn black?
Ans. Atmospheric air contains traces of H2S. It slowly reacts with silver to form silver sulphide (Ag2S) which forms a black layer over the object made of silver. Hence, the object turns black with time.
2Ag + H2S → Ag2S + H2
The white colour of oil paintings contain compounds of lead such as PbCO3, Pb(OH)2 etc. These lead compounds react with H2S present in air to form black lead sulphide (PbS). Hence, the white colour of oil paintings turn black with time.
Pb(OH)2 + H2S → PbS + 2H2O;
PbCO3 + H2S → PbS + H2CO3
Q.3 Write some of the important uses of H2S.
Ans. Some important uses of H2S gas are as follows – (1) H2S is used for the preparation of different sulphide and bisulphide salts. (2) It is used as a reducing agent in laboratories. (3) It is also used as a precipitating agent for the qualitative and quantitative analysis of inorganic salts.
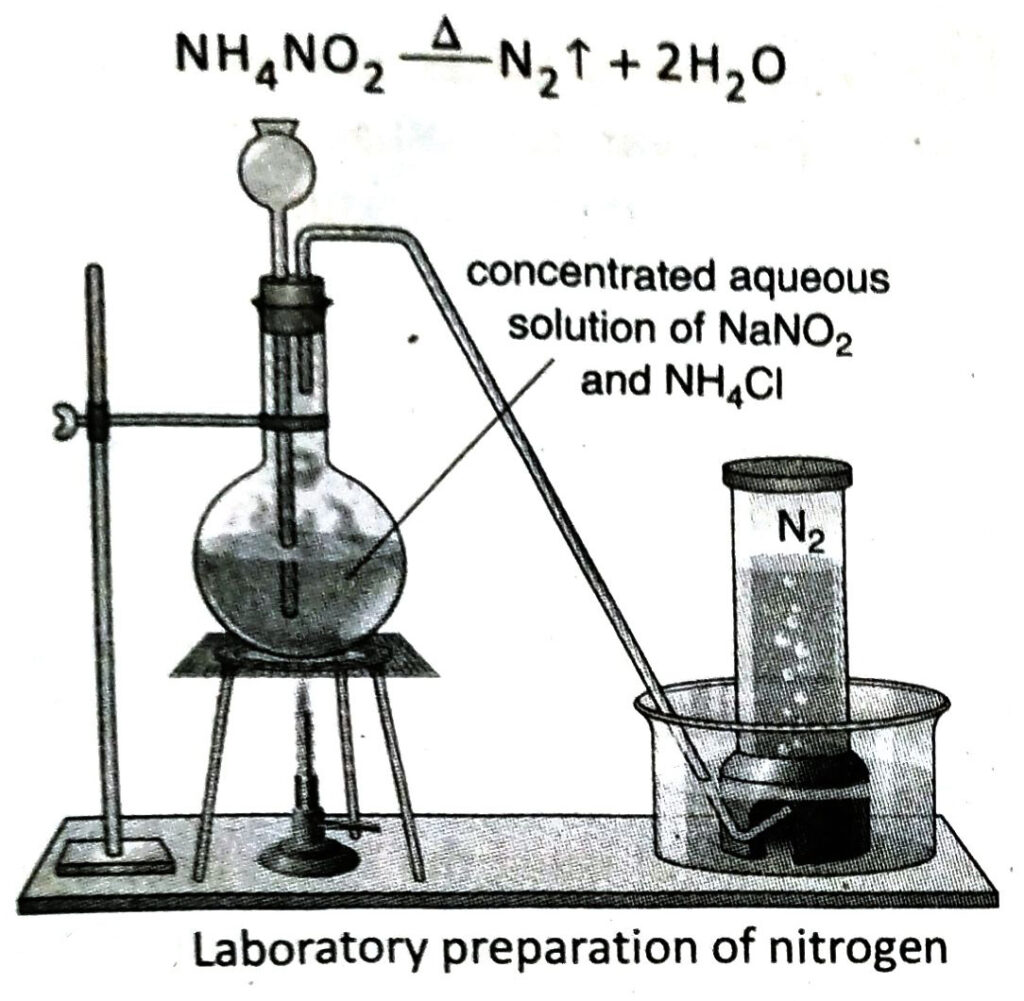
Q.4 Discuss the principle of preparation of nitrogen gas in the laboratory along with suitable equation.
Ans. Principle: In the laboratory, nitrogen gas is prepared by carefully heating a concentrated aqueous mixture of sodium nitrite and ammonium chloride taken in a 1:1 molar ratio. The reaction takes place in two steps-
(1) In the first step, ammonium chloride and sodium nitrite reacts with each other to form ammonium nitrite and sodium chloride.
NaNO2 + NH4Cl → NH4NO2 + NaCl
(2) Ammonium nitrite produced in the first step immediately decomposes to produce nitrogen on heating.
Q.5 Write some important uses of nitrogen.
Ans. Some important uses of nitrogen are-
- Nitrogen is used in the industrial preparation of ammonia and nitric acid.
- It is used in the preparation of nitrogenous fertilisers such as ammonium sulphate, ammonium phosphate, ammonium nitrate, urea, nitrolim etc.
- It is used to provide inert environment during chemical reactions.
- Liquid nitrogen is used as a refrigerant to provide very low temperatures.
Q.6 Write with chemical equations what happens when a burning Mg-ribbon is introduced into a gas jar filled with N2 gas and the product thus obtained is boiled with water.
Ans. When a red hot or a burning Mg-ribbon is introduced into a gas jar filled with dry N2 gas, it burns with a bright flame and magnesium nitride is produced.
3Mg(s) + N2(g) → Mg3N2(s)
When magnesium nitride is boiled with water, it undergoes hydrolysis to produce magnesium hydroxide and liberates pungent-smelling ammonía gas.
Mg3N2(s) + 6H2O(l) → 3Mg(OH)2(aq) + 2NH3(g)
Q.7 Discuss nitrogen fixation of aerial nitrogen by natural processes.
or, Thunderstorms and electric discharge during rainy season are helpful for the plant kingdom-explain.
Ans. During thunderstorms and electric discharge in the rainy season, atmospheric nitrogen and oxygen combine chemically with each other to form nitric oxide (NO).
This nitric oxide is then oxidised by aerial oxygen to form nitrogen dioxide.
2NO + O2 → 2NO2
NO2 thus formed dissolves in rain water to form nitrous acid (HNO2) and nitric acid (HNO3). These acids fall to the ground with rain water
2NO2 + H2O → HNO2 + HNO3
HNO3 reacts with different basic salts, such as, calcium carbonate, sodium carbonate etc., present in the soil to form water soluble nitrate salts. Plants utilise these nitrate salts for protein synthesis.
Thus, thunderstorms and electric discharge in the rainy season are helpful for the plant kingdom.
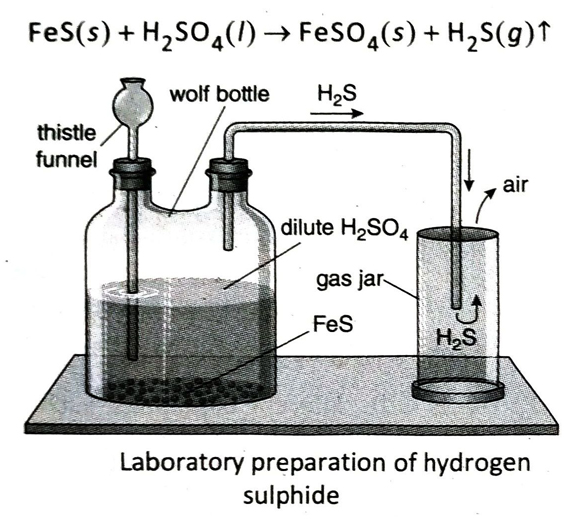
Q.8 State the principle of laboratory preparation of hydrogen sulphide and give the equation involved.
Ans. Principle: In the laboratory, hydrogen sulphide is prepared by reacting ferrous sulphide (FeS) with dilute sulphuric acid (H2SO4) at normal temperature.
Chemical equation:
Q.9 What type of gases can be prepared in the Kipp’s apparatus? What is the advantage of using the Kipp’s apparatus?
Ans. The gases which can be produced at room temperature without heating the reactants (such as, CO2, H2, H2S etc.) can be prepared in the Kipp’s apparatus. In case of such gases the reactants start reacting whenever they come in contact to each other. Moreover in order to prepare a gas in Kipp’s apparatus, one of the two reactants must be in liquid phase.
The advantage of using the Kipp’s apparatus in the laboratory is that a continuous supply of the gas, prepared in the Kipp’s apparatus, can be conveniently obtained. Moreover by using Kipp’s apparatus a gas can be prepared as per the requirement and the production of the gas can be stopped as soon as the requirement is over.
Q.10 Hydrogen sulphide is a toxic gas-explain.
Ans. Hydrogen sulphide is a colourless toxic gas with a strong smell similar to that of rotten eggs. It causes burning sensation in eyes, headache, nausea etc. On inhaling the gas, one may become unconscious and may even die on prolonged inhalation of the gas. So, inhaling air containing more than 5 ppm of H2S adversely affects our health. The toxic effect of H2S is basically due to its reaction with the essential proteins of the body cells.
Q.11 A water sample saturated with H₂S gas turns turbid when it is left in the open air for a long period of time. Why?
Ans. When a water sample saturated with H2S gas is left in the open air for a long period of time, the dissolved H2S gas is partially oxidised by aerial oxygen to form insoluble sulphur which remains in water as colloidal particles. Consequently the solution turns turbid.
2H2S + O2 → 2S↓ + 2H2O
Q.12 Why does a paper soaked in lead acetate solution turn black in presence of H2S gas?
Ans. A paper soaked in lead acetate solution turns black in presence of H2S gas because lead acetate reacts with H2S to form a black precipitate of lead sulphide. As a result, the paper turns black.
Q.13 How is hydrogen sulphide identified by an alkaline solution of sodium nitroprusside?
Ans. The gas to be identified is passed through an aqueous solution of caustic soda followed by addition of freshly prepared alkaline sodium nitroprusside solution. If the colourless solution turns violet, then it is confirmed that the unknown gas is hydrogen sulphide.
Q.14 Nitrogen gas is not produced by directly heating ammonium nitrite. Why?
Ans. Ammonium nitrite is an unstable compound and its thermal decomposition occurs very rapidly. So, if ammonium nitrite is directly heated, explosion may occur due to the high rate of the reaction. In order to avoid this, a saturated solution containing ammonium chloride and sodium nitrite is heated to produce nitrogen.
Q.15 Write some important uses of liquid nitrogen.
Ans. Liquid nitrogen can create very low temperatures, even lower than -196°C. This property of liquid nitrogen makes it useful to act as a refrigerant in preserving blood, cornea of eye etc. Nowadays, it is also used as a refrigerant for storing different food items.
Q.16 Why is nitrogen chemically inert at ordinary temperature?
Ans. In a nitrogen molecule, two adjacent nitrogen atoms are linked to each other by a triple covalent bond which is highly stable in nature. To break such a strong bond, large amount of energy is required which cannot be obtained from chemical reactions occurring at ordinary temperature. So nitrogen does not participate in chemical reactions at ordinary temperature.
Q.17 What happens when nitrogen reacts with oxygen? Give the balanced chemical equation and proper condition of the reaction.
Ans. When a mixture of nitrogen and oxygen is heated around 3000°C in an electric arc, nitric oxide (NO) is obtained as a product. The reaction is reversible and endothermic in nature.
Q.18 what happens when nitrogen reacts with hydrogen? Give the balanced chemical equation and proper condition of the reaction.
Ans. When a mixture of nitrogen and hydrogen is heated at a temperature of about 450°C under 200 atm pressure in presence of iron oxide as catalyst and a mixture of K2O and Al2O3 as promoter, ammonia is produced. The reaction is reversible and exothermic in nature.
Q.19 How is nitrolim prepared?
Ans. When nitrogen gas is passed over calcium carbide heated around 1100°C, a grey mixture of calcium cyanamide (CaNCN) and carbon is obtained. This mixture is known as nitrolim.
Q.20 How does nitrolim act as a fertiliser?
Ans. Nitrolim slowly hydrolyses to form ammonia which gets oxidised to nitrate salts by atmospheric oxygen in presence of bacteria. These nitrate salts are water soluble and are consumed by the roots of plants as food.
Q.21 Why is H2S gas collected by downward displacement of hot water instead of downward displacement of cold water?
Ans. H2S gas is soluble in cold water but insoluble in hot water. Moreover H2S is lighter than water. That is why it can be collected by downward displacement of hot water. H2S is 1.2 times heavier than air and due to this it can also be collected in the gas jar by the upward displacement of air.
Q.22 Why is N2 gas used in electric bulb instead of air?
Ans. In order to create an inert atmosphere nitrogen gas is used in electric bulbs. Air can oxidise the tungsten metal of the filament and as a result the filament burn. That is why nitrogen gas is used instead of air as nitrogen will not undergo any reaction with the filament, hence the filament will remain intact.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. Which of the following is used in the laboratory preparation of hydrogen sulphide?
A. FeSO4 and concentrated HNO3
B. FeS and concentrated H2SO4
C. FeS and dilute H2SO4
D. FeSO4 and dilute HNO3
Ans. C
2. Dilute HCl is not used in the laboratory preparation of hydrogen sulphide because,
A. it is an oxidising agent
B. it is a reducing agent
C. it reacts very slowly
D. it is a volatile substance
Ans. D
3. In aqueous solution, H2S behaves as a
A. weak monobasic acid
B. weak dibasic acid
C. weak monoacidic base
D. strong dibasic acid
Ans. B
4. The aqueous solution of which of the following salts does not produce black precipitate when H2S gas is passed through
A. ZnSO4
B. CuSO4
C. AgNO3
D. Pb(NO3)2
Ans. A
5. Combustion of H2S in inadequate supply of oxygen produces
A. SO2
B. H2SO4
C. S
D. both A and C
Ans. C
6. If HNO3 is used in the laboratory preparation of H2S, then the produced H2S gas will get oxidised to
A. SO2
B. SO3
C. H2SO4
D. S
Ans. D
7. Which of the following solutions turns violet on passing H2S gas through it?
A. alkaline sodium nitroprusside
B. sodium argentocyanide
C. Nessler’s reagent
D. potassium dichromate solution
Ans. A
8. H2S is a poisonous gas, because it
A. affects the central nervous system
B. decreases the efficiency of lungs
C. causes brain haemorrhage
D. reacts with the essential proteins of our body
Ans. D
9. When H2S gas is passed through an acidified solution of potassium dichromate (K2Cr2O7), the solution turns
A. yellow
B. green
C. black
D. orange
Ans. B
10. When H2S gas is passed through an aqueous solution of lead nitrate, the colour of the precipitate formed is
A. white
B. brown
C. green
D. black
Ans. D
11. Which of the following is not present as an impurity in N2 gas prepared in the laboratory?
A. Cl2
B. NH3
C. water vapour
D. H2S
Ans. D
12. In which of the following compounds N2 gas is trapped?
A. gun powder
B. bauxite
C. chile saltpetre
D. haematite
Ans. C
13. Nitrogen is fixed in the soil as
A. N2
B. NH3
C. metallic nitrates
D. none of these
Ans. C
14. Which of the following gases is not prepared in Kipp’s apparatus?
A. CO2
B. H2
C. O2
D. H2S
Ans. C
15. Which of the following gas is collected by downward displacement of water?
A. NH3
B. H2S
C. N2
D. HCl (g)
Ans. C
16. Nitrolim is used as a
A. fertiliser
B. explosive
C. medicine
D. none of these
Ans. A
17. Which of the following identified by its colour? gases can not be
A. Cl2
B. NO2
C. H2S
D. none of these
Ans. C
Answer in brief
1. Write the formula of hydrosulphuric acid.
Ans. The formula of hydrosulphuric acid is H2S.
2. Name the types of salts produced by H2S.
Ans. H2S can produce two types of salts – acid salts like NaHS and neutral salts like Na2S.
3. What happens when H2S gas is passed through an alkaline solution of sodium nitroprusside?
Ans. When H2S gas is passed through an alkaline solution of sodium nitroprusside, the solution turns violet.
4. Why does an orange solution of acidified potassium dichromate turns green when H2S gas is passed through it?
Ans. When H2S gas is passed through an orange solution of acidified potassium dichromate (K2Cr2O7), the later is reduced by H2S to form green coloured chromic sulphate [Cr2(SO4)3].
5. Under what conditions does H2S undergo combustion to produce powdered sulphur?
Ans. H2S undergoes combustion in limited supply of air (oxygen) to produce powdered sulphur.
6. Name the only stable hydride of sulphur.
Ans. The only stable hydride of sulphur is hydrogen sulphide (H2S).
7. In the laboratory preparation of nitrogen, the first step is the formation of NH4NO2. Which type of reaction is this?
Ans. It is a double decomposition reaction.
8. State the temperature at which calcium carbide reacts with N2 to produce nitrolim.
Ans. At 1100 °C calcium carbide reacts with nitrogen to produce nitrolim.
9. During lightning, which compound is formed due to the reaction between atmospheric N2 and O2?
Ans. During lightning, atmospheric N2 and O2 react with each other to form nitric oxide (NO).
10. Name two major gases that play a vital role in acid production.
Ans. Nitrogen (N2) and oxygen (O2) are the major gases that play a vital role in acid production.
11. Using which apparatus one can get H2S gas as and when required?
Ans. Kipp’s apparatus is used to get H2S gas as and when required.
12. Smell of which gas can be sensed from black salt?
Ans. H2S gas.
13. How does H2S gas smell like?
Ans. H2S gas smells like rotten egg.
14. Is H2S gas heavier than air or lighter than air?
Ans. H2S gas is heavier than air.
15. What is the colour of the flame when H2S burns in the air?
Ans. H2S burns in the air with a blue flame.
16. Name a gas which is reducing as well as acidic in nature.
Ans. Hydrogen sulphide (H2S) gas.
17. Write down the formula of the black precipitate formed by passing H2S gas through lead nitrate solution.
Ans. Lead sulphide (PbS).
18. Old oil paintings turn black on contact with which gas of air?
Ans. Hydrogen sulphide (H2S) gas.
19. Write down the name and colour of the precipitate obtained by passing H2S through AgNO3 solution.
Ans. Black precipate of silver sulphide (Ag2S) is formed.
20. Why do nickel or silver substances gradually turn black?
Ans. H2S, present in air reacts very slowly with nickel or silver present in these substances and forms corresponding metallic sulphides which are black in colour. This metallic sulphides form a coating on the substances. As a result they turn black.
21. Solution of which substance is mixed with aqueous solution of ammonium chloride and heated to form nitrogen at laboratory?
Ans. Aqueous solution of sodium nitrite.
22. Write down the boiling point of nitrogen.
Ans. Boiling point of nitrogen is -195.8°C.
23. How can ammonia and nitrogen gas be distinguished by their physical properties?
Ans. Ammonia gas is pungent smelling while nitrogen gas is odourless. So by this physical property these two gases can be distinguished.
24. Write down the name of the product formed by the reaction of nitrogen gas with magnesium at high temperature.
Ans. Magnesium nitride (Mg3N2).
25. Which gas can be obtained by hydrolysis of calcium cyanamide?
Ans. Ammonia (NH3)
26. What is nitrolim?
Ans. The grey mixture of calcium cyanamide and carbon.
27. Write down the use of nitrolim.
Ans. Nitrolim is used as a fertiliser.
28. Which gas is used in gas thermometer?
Ans. Nitrogen gas.
29. Mention the use of liquid nitrogen.
Ans. Liquid nitrogen is used as refrigerant to preserve cornea, eye and blood.
Fill in the blanks
1. H2S gas can be dried by using ……….. as the dehydrating agent.
Ans. P2O5
2. The poisonous nature of H2S is related to its ………… property.
Ans. chemical
3. The sulphide compound used in the laboratory preparation of H2S is ………..
Ans. FeS
4. The acid which cannot be used in the laboratory preparation of H2S is …………
Ans. HNO3
5. H2S being …………. in nature cannot be dried using ………….. dehydrating agents.
Ans. acidic, alkaline
6. H2S gas is …………. than air.
Ans. heavier
7. The basicity of hydrogen sulphide is ……………
Ans. 2
8. H2S is ………… in cold water but …………… in hot water.
Ans. soluble, insoluble
9. H2S itself is a …………….. substance but does not …………. combustion.
Ans. combustible, support
10. Zinc sulphide is …………… in colour but silver sulphide is …………… in colour.
Ans. white, black
11. H2S gas is identified by using alkaline …………. solution.
Ans. sodium nitroprusside
12. Magnesium nitride reacts with hot water to produce …………
Ans. ammonia
13. Nitric acid forms ………….. salts while react with the alkaline substances present in the soil.
Ans. nitrate
14. Solubility of nitrogen in water is very …………..
Ans. low
State whether true or false
1. Hydrogen sulphide reacts with sodium hydroxide to form sodium sulphide only.
Ans. False
2. Hydrogen sulphide gas itself is a combustible substance but not a supporter of combustion.
Ans. True
3. H2S gas reacts with a colourless aqueous solution of silver nitrate to form a black precipitate of silver sulphide.
Ans. True
4. Kipp’s apparatus is used for the preparation of those gases which are prepared in the laboratory by heating the reactants.
Ans. False
5. At ordinary temperature, nitrogen is chemically very reactive due to the presence of triple bond.
Ans. False
6. In order to remove chlorine, present as impurity, from nitrogen gas prepared in the laboratory, N2 is passed through concentrated caustic soda solution.
Ans. True
7. Nitrogen gas is adsorbed by active charcoal.
Ans. True
TOPIC – C
Industrial preparation of HCl, HNO3 and H2SO4
SHORT AND LONG ANSWER TYPE QUESTIONS
Q.1 Describe briefly the industrial preparation of hydrochloric acid.
Ans. Nowadays hydrochloric acid is commercially prepared by modern synthetic method. The major steps of the method are discussed below-
(1) Almost equal volumes of hydrogen gas and chlorine gas are taken in a combustion chamber made up of silica. When this mixture
is combusted hydrogen and chlorine combine with each other to form HCl gas.
H2(g) + Cl2(g) → 2HCl(g)
(2) The produced hydrogen chloride is cooled by passing it through a coiled tube within a condenser.
(3) The condensed gas is then absorbed in water to produce a saturated solution of hydrochloric acid.
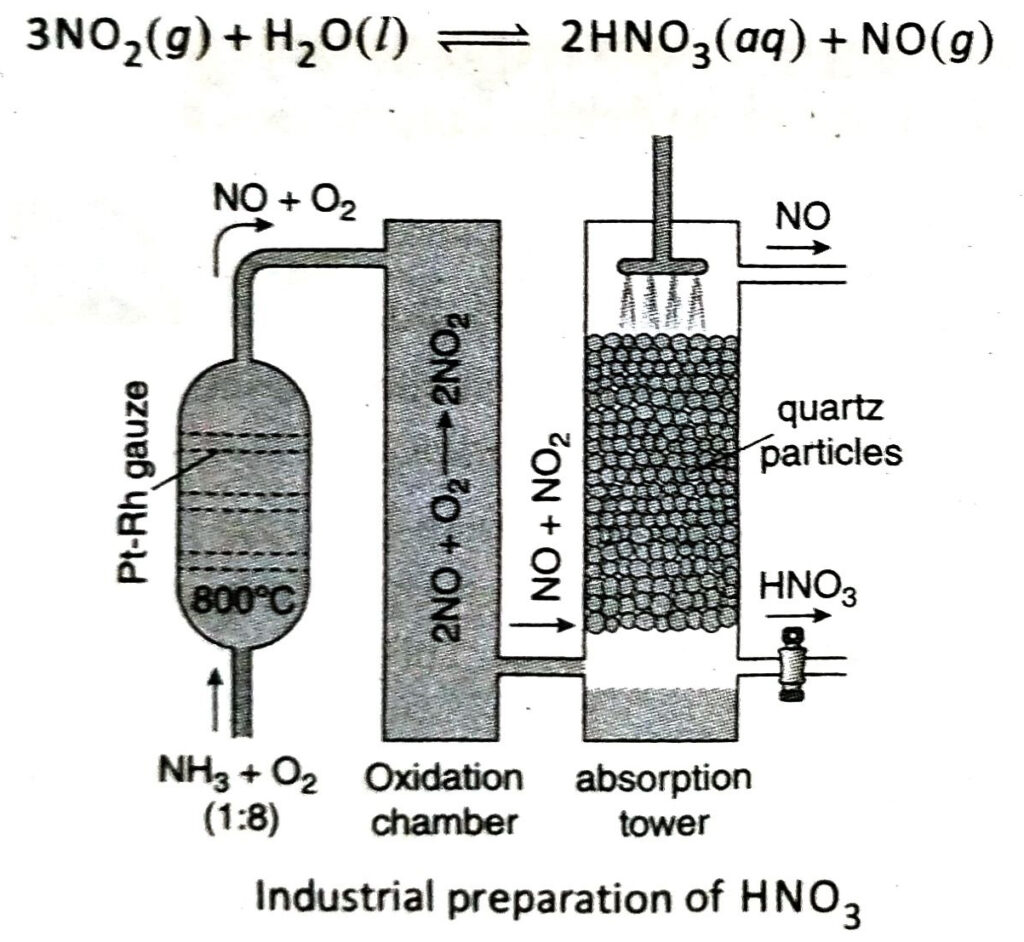
Q.2 Describe the principle of industrial preparation of nitric acid by Ostwald’s process and give the corresponding chemical equations.
Ans. The steps involved in the Ostwald’s process are as follows-
First step: A mixture of pure and dry ammonia and dust-free air taken in a ratio of 1:7.5 by volume is rapidly passed over Pt or a Pt-Rh wire gauze (which acts as a catalyst) at 800-900°C under 5-7 atm pressure. The time of contact between the gas mixture and the catalyst should not be more than 0.0014 sec. As a result, ammonia is oxidised by aerial oxygen to produce nitric oxide (NO). The reaction is reversible and exothermic in nature.
Second step: NO gas thus obtained is cooled (by bringing the temperature down to 50°C) and is then oxidised by aerial oxygen to form nitrogen dioxide (NO2).
Third step: NO2 gas thus produced is dissolved in water to produce 50% nitric acid. It is then heated to 120°C and distilled with concentrated H2SO4 to obtain 98% HNO3.
NO gas evolved in this step can be reused to prepare nitric acid.
Q.3 Why is SO3 not directly dissolved into water during industrial preparation of H2SO4 by contact process?
Ans. (1) Dissolution of SO3 in water is a very rapid and exothermic process. Consequently, it leads to an increase in temperature due to which the solubility of SO3 in water decreases and most of SO3 gas, instead of getting absorbed by water, comes out of the absorption tower. (2) At such a high temperature, water vapour and H2SO4 droplets form a kind of dense white mist which does not easily condense into liquid and causes various problems. (3) Due to the generation of large amount of heat, H2SO4 produced partially decomposes to SO3 and oxygen. Hence, production of H2SO4 gets reduced.
Q.4 Why are the catalysts used in the industrial preparation of ammonia, sulphuric acid or nitric acid taken in powdered form?
Ans. In all the cases, gaseous reactants react in presence of solid catalysts. Catalysts are taken in their powdered form in order to increase their surface area. When gases react in the presence of a solid catalyst, the gas molecules are adsorbed on the surface of the catalyst. This increases the probability of effective collisions among the reactant molecules and hence, the rate of reaction increases. Greater the surface area of the catalyst, greater will be the number of adsorbed reactant molecules on the surface of the catalyst and hence the reaction will take place at a faster rate.
Q5. Why should the mixture of ammonia and air used for the preparation of nitric acid by Ostwald’s process be pure and dust free?
Ans. In Ostwald’s process, platinum is used as the catalyst which is very expensive. If the mixture of air and ammonia is contaminated with dust, then the dust particles accumulate on the surface of the catalyst, thereby reducing its efficiency. Thus, the dust as well as impurities act as catalyst poison in this case.
Q.6 Why is the mixture of ammonia and air rapidly passed over platinum gauze catalyst for the preparation of nitric acid by Ostwald’s process?
Ans. The mixture of ammonia and air is rapidly passed over Pt-gauze catalyst for the preparation of nitric acid by Ostwald’s process. This is because the time of contact between the reactant mixture and the catalyst should not be longer than 0.0014 seconds. If the time of exposure exceeds this limit, then ammonia gets oxidised to form nitrogen instead of forming NO.
4NH3 + 3O2 → 2N2 + 6H2O
Q.7 How can we concentrate the dilute nitric acid produced by Ostwald’s method?
Ans. The dilute nitric acid (almost 50%) produced by Ostwald’s process can be first concentrated by heating it to 120°C. First of all it is concentrated up to 68% (by mass) on distillation. Then this acid is concentrated by performing distillation in presence of concentrated H2SO4 to produce 98% HNO3.
Q.8 Name the substance which is used as the catalyst in the contact process for manufacturing H2SO4. Why is the process so named?
Ans. Any one of the given compounds can be used as the catalyst in the contact process for manufacture of H2SO4 : (1) platinised asbestos or (2) vanadium pentoxide (V2O5).
As the reactants react with each other in contact with the solid surface of the catalyst, the process for the industrial preparation of H2SO4 is called contact process.
Q.9 I Why is V2O5 preferably used as the used as th catalyst in contact process?
Ans. Even though platinised asbestos is more efficient than V2O5 as a catalyst, the efficiency of the former decreases considerably in presence of even trace amount of impurities. On the other hand, V2O5 is cheaper and has less chance of getting contaminated in presence of impurities. Hence, V2O5 is preferably used as the catalyst in the contact process.
Q.10 Why is the mixture of SO2 and O2 used in the preparation of sulphuric acid by contact process, needed to be free of dust and impurities?
Ans. The mixture of SO2 and O2 used for the preparation of sulphuric acid by contact process is needed to be free of dust and impurities (mainly arsenious oxide) because there are several impurities which can act as catalyst poison and reduce the efficiency of the catalyst.
Q.11 Why is the mixture of NO and O2 gases being cooled before passing through oxidation chamber?
Ans. In Ostwald process, the mixture of NO and O2 gases is being cooled before passing through the oxidation chamber. The main objective of this measure is to reduce the probability of decomposition of the nitrogen dioxide (NO2) which is to be formed by the reaction of NO and O2 in the oxidation chamber.
Q.12 What is the advantage of being exothermic in nature of the catalytic oxidation of ammonia in Ostwald process.
Ans. In Ostwald process the catalytic oxidation of ammonia is an exothermic process, so there is no need to heat the oxidation chamber once the oxidation reaction has started. Hence the fuel for heating the oxidation chamber is thus saved.
Q.13 Convert: Iron pyrites to sulphur dioxide.
Ans. Iron pyrites can be converted to sulphur dioxide by burning it in excess air.
4FeS2 + 11O2 → 2Fe2O3 + 8SO2 ↑
Q.14 What is oleum?
Ans. Pyrosulphuric acid is commonly known as oleum. It is a light yellow oily liquid with the chemical formula of H2S2O7. It is also known as furning sulphuric acid. When sulphur trioxide is absorbed by 98% concentrated sulphuric acid, oleumn is obtained.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. Which of the following reactions is endothermic?
A. N2 + 3H2 → 2NH3
B. N2 + O2 → 2NO
C. 4NH3 + 5O2 → 4NO + 6H2O
D. 2SO2 + O2 → 2SO3
Ans. B
2. Which of the following acids reacts with skin proteins to form xanthoproteic acid?
A. sulphuric acid
B. hydrochloric acid
C. nitric acid
D. nitrous acid
Ans. C
3. Which of the following compounds is commercially manufactured by Leblanc process?
A. nitric acid
B. ammonia
C. sulphuric acid
D. soda ash
Ans. D
4. In Leblanc process, the gas produced as a by-product in the first step is
A. HCl
B. SO2
C. NH3
D. H2S
Ans. A
5. The formula of pyrosulphuric acid is
A. H2S2O8
B. H2S2O6
C. H2S2O7
D. H2S2O5
Ans. C
6. The type of reaction involved in the industrial preparation of hydrochloric acid is
A. synthesis reaction
B. substitution reaction
C. double decomposition reaction
D. decomposition reaction
Ans. A
7. In the industrial preparation of nitric acid by Ostwald’s process, the catalyst used is
A. Ni
B. Pt
C. Fe
D. Cu
Ans. B
8. The starting materials used in the industrial preparation of nitric acid by Ostwald’s process are
A. N2 and O2
B. NO and O2
C. NH3 and O2
D. N2O and O2
Ans. C
9. Optimum temperature for the 1st step of the Ostwald process of HNO3 preparation is
A. 400°C-500°C
B. 600°C-700°C
C. 700°C-800°C
D. 1000°C-1200°C
Ans. C
10. The catalyst used in the contact process of H2SO4 preparation
A. platinised asbestos
B. V2O5
C. Ni-dust
D. A or B
Ans. D
11. In which of the following phases, efficiency of the solid catalyst increases?
A. solid particle
B. fused state
C. dust
D. none of the above
Ans. C
12. The optimum temperature of the contact process for H2SO4 preparation
A. 623°C
B. 623K
C. 723°C
D. 723K
Ans. D
13. Which of the following acid is known as ‘oil of vitriol’?
A. HNO3
B. H2SO4
C. HCl
D. HBr
Ans. B
14. The oxide formed by catalytic oxidation of ammonia for the industrial preparation of nitric acid
A. NO2
B. N2O4
C. N2O5
D. NO
Ans. D
Answer in brief
1. Name the industrial process by which nitric acid is prepared from ammonia.
Ans. Industrially nitric acid is prepared from ammonia by Ostwald’s process.
2. HCl gas produces white fumes in moist air. Why?
Ans. HCl gas combines with water vapour to form tiny particles of acid which float in air producing white fumes.
3. What happens when a mixture of ammonia and air is passed over heated platinum?
Ans. When a mixture of ammonia and air is passed over heated platinum, nitric oxide (NO) and water vapour are formed.
4. Which acids are produced when NO2 gas is absorbed in water?
Ans. When NO2 gas is absorbed in water, nitric acid (HNO3) and nitrous acid (HNO2) are formed.
5. Name the process for the industrial preparation of H2SO4.
Ans. Contact process is used for the industrial preparation of H2SO4.
6. In the industrial preparation of H2SO4, which mineral of iron undergoes combustion in air to produce SO2 ?
Ans. In the industrial preparation of H2SO4, iron pyrites (FeS2) undergoes combustion in air to produce SO2.
7. What is the chemical formula of fuming sulphuric acid or oleum?
Ans. The chemical formula of fuming sulphuric acid or oleum is H2S2O7.
8. In what form catalysts are usually used in the industrial preparations of different compounds?
Ans. During industrial preparations of different compounds, catalysts are usually used in their powdered form.
9. Which inorganic acid is secreted in the stomach?
Ans. Hydrochloric acid (HCl).
10. Write down the formula of muriatic acid?
Ans. Formula of muriatic acid is HCl.
11. Which gas is evolved in the reaction of common salt with concentrated H2SO4?
Ans. Steamy fumes of hydrogen chloride (HCl) gas is evolved.
12. Write down the formula of ‘aquafortis’?
Ans. Formula of ‘aqua fortis’ is HNO3.
13. Name the industrial process for the preparation of nitric acid from ammonia.
Ans. Ostwald process.
14. Which catalyst is used in the conversion of SO2 to SO3 in contact process?
Ans. Vanadium pentoxide (V2O5).
15. Write down one use of oleum.
Ans. Oleum is used as a reagent (especially in organic reactions) in chemical laboratory.
16. What is sulphan?
Ans. Sulphan is 100% oleum. In other words sulphan or oleum is a mixture of concentrated sulphuric acid that has been saturated with excess sulphur trioxide.
Fill in the blanks
1. When an electric spark is passed through a mixture of N2 and O2 at 3000°C, ………….. is formed as the product.
Ans. NO
2. In the industrial preparation of HNO3 by Ostwald’s process, ammonia is first oxidised to …………..
Ans. NO
3. The chemical name of fuming sulphuric acid is …………..
Ans. pyrosulphuric acid
4. …………. is mixed with fuming sulphuric acid to produce sulphuric acid solution.
Ans. water
5. …………. gas is formed by the combustion of the mixture of equal volume of H2 and Cl2 gas. Pasilan Lettoniendonatio
Ans. HCl
6. …………. is used as catalyst in the industrial preparation of HNO3 by Ostwald process.
Ans. Platinum (Pt)
7. …………. acid is prepared by contact process.
Ans. Sulphuric
8. The conversion of SO2 to SO3 by contact process is ………… and …………. is nature.
Ans. reversible, exthermic
9. The formula of pyrosulfuric acid is …………..
Ans. H2S2O7
State whether true or false
1. The anhydride of nitric acid is nitrogen pentoxide (N2O5).
Ans. True
2. The oxidation of SO2 to SO3 in the contact process is carried out at a temperature of 450°C under 1.5 atm pressure.
Ans. True
3. The first step of the contact process involves reduction of iron pyrites to form sulphur dioxide.
Ans. False
4. The industrial preparation of HCl involves combustion of a mixture of equal volumes of hydrogen and chlorine gases.
Ans. True
5. In contact process of H2SO4 preparation, SO3 is directly dissolved in water to form sulphuric acid.
Ans. False