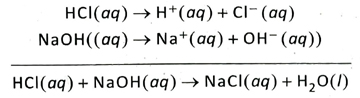WBBSE 9th Class Science Solutions Physical Science & Environment Chapter – 4.4 Acids, Bases and Salts
West Bengal Board 9th Class Science Solutions Physical Science & Environment Chapter – 4.4 Acids, Bases and Salts
WBBSE 9th Class Physical Science & Environment Solutions
Synopsis
- According to Arrhenius theory of electrolytic dissociation, an acid is a hydrogen-containing compound which dissociates in aqueous solution to produce H+ ion (or H3O+ ion) as the only cation. On the other hand, compounds that yield OH– ions as the only anion in their aqueous solutions are called bases.
- Acids like H2SO4, HCl, HNO3 and bases like NaOH, KOH find important applications in different industries.
- Metals do not produce hydrogen on reacting with cold and very dilute HNO3. Only Mg and Mn can displace hydrogen from cold and very dilute nitric acid.
- Hydrochloric acid is identified by using aqueous solution of silver nitrate. Aqueous solution of barium chloride is used to identify sulphuric acid. Nitric acid is identified by the ring test.
- Strong acids and strong bases are highly corrosive in nature. So, proper precautionary measures are adopted while using them.
- When concentrated nitric acid is refluxed with small amount of starch, a yellow coloured solution of nitric acid containing different oxides of nitrogen is obtained. It is called fuming nitric acid.
TOPIC – A
Concept of Acid and Base
SHORT AND LONG ANSWER TYPE QUESTIONS
1. Define acids in the light of Arrhenius theory of electrolytic dissociation.
Ans. According to Arrhenius theory of electrolytic dissociation, an acid is defined as a hydrogencontaining compound that dissociates in water to produce H+ ions (or H3O+ ions) as the only cation. For example, HCl ionises in water to produce H+ (or H2O+) ions as the only cation. Hence, HCl is an acid.
HCl → H+ + Cl– ; H+ + H2O → H3O+
2. Mention the general properties of acids.
Ans. General properties of acids are-
- Acids are ionised in aqueous solution forming H ion (H3O⊕ ion actually) and thus can conduct electricity.
- Acids are sour in taste
- Acids turn blue litmus paper red.
- Acids generally produce hydrogen gas in reaction with metal.
- Acids generally produce carbon dioxide in reaction with carbonates and bicarbonates.
- Acids produce salt and water in reaction with alkali.
3. Why do free H+ ions not exist in water or aqueous solution?
Ans. H+ ions combine with electronegative oxygen atoms in water to produce hydroxonium or hydronium ions (H3O+). So, free H+ ions do not exist in water or aqueous solution.
4. What are inorganic or mineral acids and give some examples.
Ans. Acids which are obtained from minerals or produced from inorganic substances are known as mineral acids. Hydrochloric acid (HCl), sulphuric acid (H2SO4), nitric acid (HNO3) are some examples of mineral acids.
5. What are organic acids and give some examples.
Ans. Acids (containing carbon atoms) which are obtained from plants or animals are called organic acids. For example, formic acid is found in ant venom, lemon contains citric acid, lactic acid is present in curd etc.
6. What is meant by ionisation of an acid in aqueous solution and give example.
Ans. In aqueous solution, an acid dissociates to form H+ ions which are unstable in nature. So these ions combine with water molecules to form hydronium ions (H3O+). This decomposition of an acid in its aqueous solution is known as the ionisation of an acid.
7. What are weak acids and give examples.
Ans. Acids which partially dissociate in aqueous solution to produce small amount of H3O+ ions and most of the molecules remain undissociated in the solution are called weak acids. Some examples are acetic acid (CH3COOH), carbonic acid (H2CO3), citric acid etc.
8. Mention the general properties of bases.
Ans. General properties of bases are-
- Bases form OHΘ ion as anion when they are dissolved in water.
- Bases taste bitter and are generally soapy in nature.
- Bases turn red litmus paper blue.
- Bases form salt and water in reaction with acids.
9. Mention the limitations of Arrhenius acid-base concept.
Ans. The limitations of Arrhenius acid-base concept are as follows-
- According to this concept, an acid or a base is defined on the basis of their dissociation in aqueous solutions. Thus, the concept is limited to aqueous medium only. It cannot explain the acidic or basic property of compounds in solvents other than water (non-aqueous solvents) like liquid ammonia, benzene etc.
- According to this concept, a base must yield OH ions in aqueous medium. Thus, the theory fails to explain the basic nature of compounds like ammonia (NH3), methyl amine (CH3NH2), aniline (C6H5NH2) etc. Similarly acidic nature of compounds like BF3 or AlCl3 cannot be explained by this theory as these compounds do not produce H+ ions in aqueous medium.
10. All acids are hydrogen-containing compounds, but all hydrogen-containing compounds are not acids-justify the statement with example.
Ans. According to Arrhenius theory, an acid must contain H-atom. However, a compound containing hydrogen will be called an acid only if it has the following properties-
- It will dissociate in aqueous solution to produce H3O+ (hydroxonium) ions.
- It will produce hydrogen gas on reacting with metals.
- It will react with a base or an alkali to produce salt and water.
- Its aqueous solution will turn blue litmus red.
However there are compounds containing hydrogen, which do not exhibit these properties. For example
- Compound like PH3, NH3, CH4, sugar (C₁2H22O11) do not yield H3O+ ions in aqueous solution. Neither do they react with metals to produce hydrogen gas nor do they react with bases to form salt and water.
- Sodium reacts with H2O to produce hydrogen gas but the reaction produces NaOH instead of a salt.
- Al, Zn etc. react with compounds like sodium hydroxide, potassium hydroxide etc. to produce hydrogen gas but these compounds do not yield H3O+ ions in their aqueous solution. So, these compounds are not acids.
11. State whether Al(OH)3 is a base or an 1) is a base or an alkali. Justify your choice.
Ans. Water soluble metal hydroxides are known as alkalis. Aluminium hydroxide [Al(OH)3] is a base as it reacts with acid to produce salt and water but it is insoluble in water. So, aluminium hydroxide is not an alkali.
12. Give an example of a reaction where two gases react to form a solid.
Ans. Ammonia gas reacts with hydrogen chloride gas to form fine particles of solid ammonium chloride which floats in air forming white fumes.
Equation: NH3 + HCl → NH4Cl
13. Mention some uses of hydrochloric acid in industries.
Ans. Some important uses of hydrochloric acid are as follows-
- In the preparation of chlorine and different metal chlorides.
- Preparation of aqua regia and glucose (from starch) and in removing scales from boilers.
- In dye industries, tanneries, pharmaceutical industries and during galvanisation and tinplating of iron.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. A covalent compound whose aqueous solution is acidic is
A. CH4
B. CCl4
C. HCl
D. NH3
Ans. C
2. A covalent compound whose aqueous solution is alkaline is
A. CH4
B. CCl4
C. HCl
D. NH3
Ans. D
3. One of the limitations of Arrhenius acid-base theory is that
A. it is applicable only for organic solvents
B. it is applicable only for inorganic solvents
C. water is essentially required as the solvent
D. no solvent is required
Ans. C
4. In aqueous solution H+ ion is present as
A. H+ ion
B. H2+ ion
C. H3O+ ion
D. H2O– ion
Ans. C
5. Which of the following is used to prepare soaps and detergents?
A. HNO3
B. HCl
C. H2SO4
D. NaOH
Ans. D
6. Which of the following is known as ‘aqua fortis’?
A. NaOH
B. HNO3
C. HCl
D. H2SO4
Ans. B
7. Which of the following is called the ‘king of chemicals’?
A. NaOH
B. HNO3
C. HCl
D. H2SO4
Ans. D
8. The acid used in the preparation of starch from glucose is
A. HCl
B. HNO3
C. H2SO4
D. H2PO4
Ans. A
9. Aqua regia is a
A. 3:1 mixture of concentrated HCl and concentrated HNO3
B. 1:3 mixture of concentrated HCl and concentrated H2SO4
C. 2:3 mixture of HNO3 and H2SO4
D. 1:1:1 mixture of HCl, HNO3 and H2SO4
Ans. A
10. Which of the following does not produce hydrogen on reacting with dilute acids?
A. Fe
B. Zn
C. Cu
D. Mg
Ans. C
11. Which of the following can produce hydrogen by reacting with cold and very dilute HNO3 ?
A. Fe
B. Zn
C. Cu
D. Mg A Fe
Ans. D
Answer in brief
1. For which property of water, an acid ionises in water to produce H3O+ ions?
Ans. Due to the polar nature of water, an acid ionises in water to produce H3O+ ions.
2. Give some examples of organic acid.
Ans. Acetic acid (CH3COOH), formic acid (HCOOH) etc., are some examples of organic acid.
3. Name an inorganic acid which is synthesised in human body.
Ans. Hydrochloric acid (HCl).
4. Give an example (name and formula) of a weak acid.
Ans. Acetic acid (CH3COOH).
5. Name two acids used in daily life.
Ans. Examples of acids used in daily life are-
(1) acetic acid (vinegar is used in cooking),
(2) hydrochloric acid (muriatic acid is used to clean bathrooms).
6. Name a base which is not a metallic oxide or hydroxide.
Ans. Ammonia (NH3).
7. Name two basic substances used in daily life.
Ans. Washing soda (Na2CO3) and baking soda (NaHCO3) are two basic substances used in daily life.
8. Name a metal which cannot produce hydrogen gas by reacting with dilute mineral acids.
Ans. Copper.
9. Give example of a volatile mineral acid.
Ans. Hydrochloric Acid (HCl).
10. Name a covalent gaseous compound which ionises in water and acts as a strong acid.
Ans. Hydrogen chloride (HCl) is a gaseous covalent compound which ionises in water and acts as a strong acid.
11. What is muriatic acid?
Ans. Muriatic acid is hydrochloric acid (HCl).
12. Which metals can displace hydrogen from cold and dilute nitric acid?
Ans. Metals such as, magnesium (Mg) and manganese (Mn) can displace hydrogen from cold and dilute nitric acid.
13. Which acid is used to remove impurities from gold?
Ans. Nitric acid (HNO3).
14. State the major use of aqua regia.
Ans. Aqua regia is used to dissolve noble metals like gold, silver, platinum etc.
15. Which acid is used in the preparation of TNT?
Ans. Nitric acid (HNO3).
16. Which gas is excreted from jewellery shops?
Ans. Nitrogen dioxide (NO2).
17. Which acid is used more in jewellery industry?
Ans. HNO3 (nitric acid).
18. Which acid is used to prepare nitroglycerine?
Ans. Nitric acid is used to prepare nitroglycerine.
19. Which acid is used in the ring test for identification of nitric acid?
Ans. Sulphuric acid.
20. Which acid is used in the preparation of inorganic fertiliser, ammonium sulphate?
Ans. Sulphuric acid (H2SO4).
21. What is oil of vitriol?
Ans. Sulfuric acid is termed as oil of vitriol.
Fill in the blanks
1. The formula of hydronium ion is …………
Ans. H3O+
2. A base is a compound which dissociates in water to produce ………….. ions.
Ans. OH–
3. Ammonia is a base because it reacts with acids to produce …………
Ans. salts
4. Fuming nitric acid is a strong …………… agent.
Ans. oxidising
5. ………… is used in the preparation of picric acid.
Ans. HNO3
6. …………. is used to absorb water vapour.
Ans. H2SO4
7. The base used to prepare synthetic fibre or rayon is …………
Ans. NaOH
8. Copper reacts with hydrochloric acid in presence of …………
Ans. O2
9. The metal which is not used in the preparation of hydrogen is …………
Ans. Cu
10. Freshly prepared solution of …………. is required for the ring test.
Ans. FeSO4
State whether true or false
1. Water soluble metal hydroxides are called alkalis.
Ans. True
2. The compound responsible for the formation of brown ring in the ring test is pentaaquanitrosyliron (II) sulphate.
Ans. True
3. Prolonged secretion of acid in the stomach causes ulcer.
Ans. True
4. Hydrochloric acid exhibits reducing property while nitric acid exhibits oxidising property.
Ans. True
5. The basicity of H2SO4 is 1.
Ans. False
6. Nitric acid is extensively used in the manufacture of explosives.
Ans. True
7. HCl is the organic acid produced in human body.
Ans. False
8. BaCl2 solution is used to identify HNO3.
Ans. False
TOPIC – B
Qualitative Concept of pH
SHORT AND LONG ANSWER TYPE QUESTIONS
1. What is meant by pH of a solution?
Ans. pH of a solution is defined as the negative logarithm (to the base 10) of the molar concentration of H3O+ ions in the solution. pH comes from the German word Potenz de Hydrogen where Potenz means power.
where [H3O+] = molar concentration of H3O+ ions in the solution.
2. What i idea is obtained about the nature out the nature a solution fr of a from its pH value?
Ans. At 25°C, if the pH of a solution is less than 7, then the solution is acidic in nature. If the pH is greater than 7, then the solution is alkaline in nature whereas if the pH is equal to 7, then the solution is a neutral solution.
3. What is pH-paper? How is it used to determine the pH of a solution?
Ans. A pH-paper is a paper coated with universal indicator which is used to get an idea about the pH of a solution.
To determine the pH of a solution, the pH-paper is dipped into the solution. The paper attains a definite.colour depending on the pH of the solution. The colour of the pH-paper is then compared with the standard pH colour strip and consequently, the pH of the solution is determined.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. The hardest part of our body is
A. enamel of teeth
B. bones
C. skin
D. nails
Ans. A
2. The pH of two solutions, A and B are 3 and 6 respectively. It means that
A. solution A is twice as acidic as solution B
B. solution B is twice as acidic as solution A
C. solution A is 1000 times more acidic than solution B
D. solution B is 1000 times more acidic than solution A
Ans. C
3. The chemical nature of commonly used toothpastes is
A. acidic
B. neutral
C. alkaline
D. amphoteric
Ans. C
4. pH of blood is
A. 4
B. 5
C. 7
D. 7.35-7.45
Ans. D
5. pH of milk is
A. 5.5-5.6
B. 3.4-3.7
C. 6.5-6.7
D. 8.5-9.5
Ans. C
6. If the H+ ion concentration of a solution is 10-5 g-ion/litre, then the pH of the solution will be
A. 10
B. 5
C. 3
D. 7
Ans. B
7. The pH at which the enamel of teeth begins to decay is
A. 3.5
B. 4.5
C. 5.5
D. 6.5
Ans. C
8. Due to acid rain, the pH of agricultural lands
A. increases
B. decreases
C. remains unchanged
D. increases in some places and decreases in other places
Ans. B
9. The range of the pH scale at 25°C is
A. 0-10
B. 0-14
C. 0-12
D. 1-14
Ans. B
10. If acid is added to pure water, pH of the medium will
A. increase
B. decrease
C. remain same
D. be zero
Ans. B
11. If a litmus paper is dipped in an alkaline solution, its colour will be
A. blue
B. red
C. yellow
D. green
Ans. A
12. Which of the solutions has the highest pH ?
A. caustic soda
B. milk of magnesia
C. vinegar
D. formic acid
Ans. A
13. If a solution turns reddish-pink on addition of phenolphthalein, its pH may be
A. 4
B. 7
C. 10
D. 5
Ans. C
14. Concept of pH scale was given by
A. Arrhenius
B. Sorensen
C. Rutherford
D. Lewis
Ans. B
15. pH of an alkaline solution may be
A. 5.4
B. 6.2
C. 7
D. 9.2
Ans. D
Answer in brief
1. What is the concentration of H⊕ ion in pure water at 25°C?
Ans. 10-7 mol L-1.
2. In which solvent between water and benzene, an acid does not undergo ionisation?
Ans. An acid does not undergo ionisation in benzene because it is a non-polar solvent.
3. What is the nature of an aqueous solution having pH = 4?
Ans. Acidic in nature.
4. What is the nature of an aqueous solution having pH = 7?
Ans. Neutral in nature.
5. What is the nature of an aqueous solution having pH = 9?
Ans. Alkaline in nature.
6. What is the value of pH of pure water at 25°C ?
Ans. The pH of pure water at 25°C is 7.
7. What is the pH of rain water?
Ans. The pH of rain water is generally 5.6.
8. Odd one out: litmus paper, pH paper, methyl orange, phenolphthalein.
Ans. pH paper.
9. What is the major component of tooth enamel?
Ans. Calcium phosphate [Ca3(PO4)2].
10. Why does acid form at the base of the tooth?
Ans. The residual food particles, especially the sugar particles get stuck to the space between the teeth and are dissociated by bacteria to form acid. Hence the statement.
11. Which substance should be used to make alkaline agricultural land suitable for farming?
Ans. Compost fertilizer is generally used.
Fill in the blanks
1. Scientist ……….. introduced the pH scale.
Ans. Sorensen
2. The term pH originates from the German word ………..
Ans. Potenz de Hydrogen
3. At 25°C, the maximum pH value of a dilute solution is ………..
Ans. 14
4. pH is the negative logarithm of ……….. ion concentration in a solution.
Ans. H3O+
5. Blood is slightly ……….. in nature.
Ans. alkaline
State whether true or false
1. The pH of an aqueous solution is 3. The solution is alkaline in nature.
Ans. False
2. If the H3O+ ion concentration of an aqueous solution is 1 × 10-5 mol · L-1, then the pH of the solution will be 5.
Ans. True
3. Saliva is acidic in nature.
Ans. True
4. pH of pure water is 7 at any temperature.
Ans. True
TOPIC – C
Acidic, Basic, Amphoteric Oxides and Acid Rain
SHORT AND LONG ANSWER TYPE QUESTIONS
1. Chromium trioxide is an acidic oxide though it is a metallic oxide. Explain.
Ans. Chromium trioxide (CrO3) dissolves in water to produce chromic acid (H2CrO4). Hence, it is an acidic oxide.
CrO3 + H2O → H2CrO4
2. What are acidic oxides and give examples.
Ans. The oxides which react with bases to form salt and water are known as acidic oxides. Generally oxides of non-metals are acidic oxides. Some examples of acidic oxides are carbon dioxide (CO2), sulphur dioxide (SO2), phosphorus pentoxide (P2O5) etc.
S + O2 → SO2 ; SO2 + 2NaOH → Na2SO3 + H2O
3. Define basic oxides with examples.
Ans. The oxides which react with acids to form salt and water are known as basic oxides. Generally oxides of metals are basic oxides. Some examples are calcium oxide (CaO), ferrous oxide (FeO), magnesium oxide (MgO) etc.
2Ca + O2 → 2CaO ; CaO + 2HCl → CaCl2 + H2O
4. Define amphoteric oxides with examples.
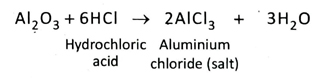
Ans. The oxides which react with both acids and bases to form salt and water are called amphoteric oxides. Some examples are, zinc oxide (ZnO), aluminium oxide (Al2O3) etc.
ZnO + 2HCl → ZnCl2 + H2O
ZnO + 2NaOH → Na2ZnO2 + H2O
5. Why is aluminium oxide called an amphoteric oxide?
Ans. Aluminium oxide reacts with both acids and bases separately to form salt and water.
So, aluminium oxide is known as an amphoteric oxide.
6. Classify the following oxides: ZnO, SO2, P2O5, K2O, Al2O3.
Ans. Acidic oxides: SO2, P2O5
Basic oxides: Na2O, K2O
Amphoteric oxides: ZnO, Al2O3.
7. What are acid anhydrides?
Ans. The oxide of a non-metal (acidic oxide) which dissolves in water to form its corresponding acid is called an acid anhydride. For example, phosphorus pentoxide (P2O5) dissolves in water to form phosphoric acid (H3PO4). Thus, phosphorus pentoxide is the anhydride of phosphoric acid.
P2O5 + 3H2O → 2H3PO4
8. Define mixed acid anhydride with example.
Ans. If a non-metallic oxide dissolves in water to produce more than one acid, then the oxide is considered as the anhydride of both the acids and is called a mixed acid anhydride. For example, NO2 dissolves in cold water to produce nitrous acid (HNO2) and nitric acid (HNO3). Hence, NO2 is a mixed acid anhydride.
2NO2 + H2O → HNO2 + HNO3
9. What is acid rain?
Ans. Normal rain water is slightly acidic. However, if rain water contains excess amount of acids like H2SO4, HNO3 and HCl, then the pH of rain water ranges between 3.5-5.6. This is known as acid rain.
10. What are the causes of acid rain?
Ans. Different causes of acid rain can be classified into two categories-natural and man-made causes. Acidic oxides emitted due to natural causes and different human activities mix with the atmospheric water vapour to cause acid rain.
- Natural causes: (1) SO2 gas is released into the atmosphere during volcanic eruptions. (2) Nitrogen and oxygen present in air combine to form different oxides of nitrogen NOx during lightning discharges. (3) Bacterial decomposition of ammonium salts present in soil also produces oxides of nitrogen (NOx) that are released into the atmosphere.
- Man-made causes: (1) Different oxides of sulphur and nitrogen are released into the atmosphere due to combustion of fossil fuels like coal and petroleum used in automobiles, thermal power plants, oil refineries, metal extraction industries etc. (2) Huge amounts of HCl gas is released from factories where hydrochloric acid is extensively used.
These gases react with atmospheric oxygen, ozone and water vapour to produce different acids which float in air in the form of aerosols and come down on the earth’s surface along with rain water.
11. What is stone cancer?
Ans. Scars and pits formed on the surface of buildings, sculptures, memorials and monuments made of marble due to acid rain are collectively called stone cancer. The scars are formed due to the reaction of the acid with marble (calcium carbonate, CaCO3).
12. Discuss the harmful effects of acid rain.
Ans. The harmful effects of acid rain are as follows-
- Effect on soil and vegetation: Acid rain increases acidity of soil. The increased acidity changes the solubilities of different metal salts in the soil. Consequently, fertility of the soil decreases which reduces the agricultural productivity. Photosynthetic activities of the plants get dis rupted due to acid rain. Soil microorganisms and other living organisms of the soil are also adversely affected due to acid rain.
- Effect on aquatic plants and animals: Acid rain decreases normal pH of different water bodies which in turn decreases reproductive capacity of fish leading to decresed production of spawns. Excessive increase in the acid level of water kills the flora and fauna of the water body and disturbs the marine ecosystem.
- Effect on human beings: Due to acid rain, some metals dissolve in rain water to form toxic salts which on entering the human body cause harmful effects. Acid rain damages our skin, hair and body cells. Acids like H2SO4 and HNO3 present in acid rain enter the human body and adversely affect our nervous system, respiratory system and digestion process.
- Effect on sculptures and monuments: Acid rain causes extensive damage to buildings, memorials, monuments and sculptures made of marble. Marble (CaCO3) reacts with acids and forms insoluble calcium sulphate that deposits on the surfaces of the buildings. This causes scars, pits on the surface and erodes it.
CaCO3 + H2SO4 → CaSO4 ↓ + CO2 ↑ + H2O
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. An example of an acidic oxide is
A. CaO
B. Na2O2
C. CO2
D. MgO
Ans. C
2. An example of a basic oxide is
A. CO2
B. NO2
C. CaO
D. SO2
Ans. C
3. An example of an amphoteric oxide is
A. ZnO
B. CaO
C. MgO
D. FeO
Ans. A
4. Acid rain has adversely affected
A. Taj Mahal
B. Victoria Memorial
C. sculptures made of marbles
D. all of these
Ans. D
5. As a consequence of acid rain, pH of soil
A. increases
B. decreases
C. remains unaltered
D. sometime increases, sometime decreases
Ans. B
6. Which of the following can form each of acidic, basic and amphoteric oxide?
A. S
B. Pb
C. Ca
D. Cr
Ans. D
7. The gas released from Mathura oil refinery which is the reason behind the weathering of the Taj Mahal is
A. CO2
B. CH4
C. CO
D. SO2
Ans. D
8. Number of amphoteric oxides among Cr2O3, ZnO, PbO, Al2O3 is
A. 1
B. 2
C. 3
D. 4
Ans. D
9. The gas responsible for acid rain is
A. CH4
B. NO2
C. N2O
D. O3
Ans. B
10. Which of the following elements form acidic oxide?
A. Na
B. Mg
C. P
D. Al
Ans. C
11. N2O5 is
A. acidic oxide
B. basic oxide
C. amphoteric oxide
D. neutral oxide
Ans. A
12. Which one is not an acid anhydride?
A. CO2
В. СО
C. N2O5
D. SO3
Ans. B
13. Which one of water, carbon dioxide, calcium oxide and nitrogen dioxide is a neutral oxide?
A. water
B. carbon dioxide
C. calcium oxide
D. nitrogen dioxide
Ans. A
Answer in brief
1. Name some metallic oxides which exhibit acidic property in water.
Ans. Manganese heptoxide (Mn2O7), chromium trioxide (CrO3) etc.
2. Give some examples of acidic oxide.
Ans. Some examples of acidic oxide are carbon dioxide (CO2), sulphur trioxide (SO3) etc.
3. Name two acidic metallic oxide.
Ans. Manganese heptoxide (Mn2O7) and chromium trioxide (CrO3).
4. Which type of oxide is P2O5?
Ans. P2O5 is an acidic oxide.
5. Which class of oxides does calcium oxide belong to?
Ans. Calcium oxide belongs to the class of basic oxide.
6. Give some examples of amphoteric oxide.
Ans. Some examples of amphoteric oxide are zinc oxide (ZnO), aluminium oxide (Al2O3) etc.
7. Which acid is formed when nitrogen dioxide (NO2) gas dissolves in water?
Ans. Nitric acid (HNO3) and nitrous acid (HNO2).
8. Which acid is formed when sulphur dioxide (SO2) gas dissolves in water?
Ans. Sulphurous acid (H2SO3).
9. Which gas dissolves in water to produce sulphuric acid?
Ans. Sulphur trioxide (SO3).
10. Give an example of neutral oxide.
Ans. Water (H2O).
11. Give example of a base which is neither metallic oxide nor metallic hydroxide.
Ans. Ammonia (NH3).
12. What is the pH range of acid rain?
Ans. pH range of acid rain is 3.5 to 5.6.
13. Write down the names of two gases responsible for acid rain.
Ans. SO2 and NO2 gas.
Fill in the blanks
1. The corrosion of marble buildings and monuments caused by acid rain is known as …………..
Ans. stone cancer
2. An amphoteric oxide reacts with both …………. and ………… to produce salts and water.
Ans. acids, bases
3. …………. oxides are generally acidic in nature.
Ans. Non-metallic
4. ………… oxides are generally basic in nature.
Ans. Metallic
5. An acid present in acid rain is …………
Ans. H2SO4 or HNO3
6. SO2 dissolves in water to produce …………. acid.
Ans. sulphurous
7. The product formed due to oxidation of SO2 dissolves in water to produce …………. acid.
Ans. sulphuric
8. ………. is an example of mixed acid anhydride.
Ans. NO2
9. Aluminium oxide is an example of ………… oxide.
Ans. amphoteric
10. Fe3O4 is an example of ……….. oxide.
Ans. mixed
11. Example of neutral oxide is …………..
Ans. H2O
State whether true or false
1. Nitrogen dioxide dissolves in water to form nitric acid only.
Ans. False
2. Acid rain mainly consists of the acids like HCl, H2SO4 and CH3COOH.
Ans. False
3. CO is an acidic oxide.
Ans. False
4. Example of an amphoteric oxide is Al2O3.
Ans. True
5. PbO is an amphoteric oxide.
Ans. True
6. CO is dissolved in acid rain.
Ans. False
7. NO is an acidic oxide.
Ans. False
TOPIC – D
Neutralisation, Indicator, Antacids, Salts and Their Classification
SHORT AND LONG ANSWER TYPE QUESTIONS
1. What are acid-base indicators? Name two acid-base indicators.
Ans. The chemical substances which indicate the end point of an acid-base neutralisation reaction by changing their colour are called acid-base indicators.
Two widely used acid-base indicators are methyl orange and phenolphthalein.
2. What changes will be observed in the colour of a litmus paper when it comes in contact with dry ammonia gas and aqueous solution of ammonia?
Ans. No change will be observed in the colour of litmus paper when it comes in contact with dry ammonia gas. However, the litmus paper turns blue in aqueous solution of ammonia.
3. What is acid-base neutralisation reaction?
Ans. Equivalent amount of an acid reacts quantitatively with equivalent amount of a base to produce salt and water and as a result, both the acid and the base lose their individual properties. This reaction is known as acid-base neutralisation reaction. For example, 1 gram-equivalent HCl. reacts with 1 gram-equivalent NaOH to produce salt and water. Both HCl and NaOH lose their individual properties in course of this reaction.
4. What is neutralisation point? How is neutralisation point determined?
Ans. In an acid-base reaction, the point at which the acid and the base quantitatively react with each other to produce salt and water and as a result, the solution becomes neutral is known as neutralisation point.
The neutralisation point of an acid-base reaction is identified by using an indicator. An indicator indicates the neutralisation point by changing its colour.
5. How will you differentiate between Na2CO3 and NaHCO3 solution with one drop of an indicator?
Ans. Both Na2CO3 and NaHCO3 hydrolyse in their aqueous solutions to form NaOH thereby making the resulting solution alkaline. However, the pH value of Na2CO3 solution is greater than that of NaHCO3. Hence, on addition of one drop of phenolphthalein to Na2CO3 solution, the solution turns On the other hand, if one drop of phenolphthalein is added to NaHCO3 solution, the solution remains colourless. Hence one drop of phenolphthalein is sufficient to differentiate between Na2CO3 solution and NaHCO3 solution.
6. Mention the characteristic features of indicators used in neutralisation reactions? Mention the criteria for choosing an indicator for neutralisation reaction with example.
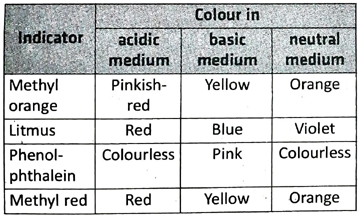
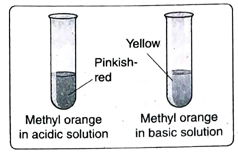
Ans. An acid-base indicator should be such that it can exhibit different colours in acidic, basic and neutral solutions. For example, methyl orange is yellow in alkaline solution, pinkish-red in acidic solution and orange in neutral solution.
An acid-base indicator is chosen for a particular neutralisation reaction on the basis of the change in pH-value of the solution at the equivalence point or end point. The selected indicator must exhibit a sharp change in colour in the same pH range as required around the end point. For example, in the neutralisation of HCI and NaOH, a sharp change in pH of the solution from 3.34 to 9.7 is observed around the end point. The pH range of methyl orange is 3.4-4.4. Hence, methyl orange can be used for the neutralisation reaction of HCI and NaOH.
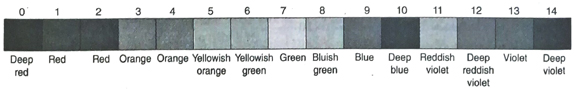
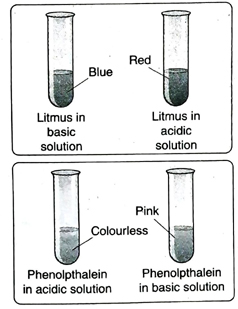
7. Tabulate the colours exhibited by the following indicators in acidic, basic and neutral medium-methyl orange, litmus, phenolphthalein and methyl red.
Ans. The colours exhibited by the given indicators in acidic, basic and neutral medium are tabulated below-
8. How is an indicator chosen for an acidbase neutralisation reaction?
Ans. At the end point of an acid-base neutralisation reaction, there is a rapid and sharp change in the pH of the solution. The indicators which can change their colour in that pH-range are selected for those acid-base neutralisation reactions. For example, in the neutralisation of a strong acid and a strong base, the range of pH change is maximum near the end point. The pH range of almost all the indicators lies in this range. Hence, any indicator is suitable for titration of a strong acid and a strong base. On the other hand, in the neutralisation of a weak acid and a weak base, the range of pH change is minimum near the end point. No suitable indicator having pH range in this region has been found. Hence, the titration of a weak acid and a weak base is not possible.
9. Mention the colour of the solutions if phenolphthalein is added to them. (1) NaCl (2) Ca(OH)2 (3) HCl (4) MgCl2.
Ans.
| Solution of the compound |
Colour on addition of phenolphthalein |
| 1. NaCl |
Colourless |
| 2. Ca(OH)2 |
Redish pink |
| 3. HCl |
Colour less |
| 4. MgCl2 |
Colour less |
10. what are antacids? Give some examples.
Ans. The substances which are used to neutralise the excess hydrochloric acid secreted in the stomach and maintain the pH of the gastric juice at an optimum level, are known as antacids.
Gelusil, Digene, Diovol are some of the commonly used antacids.
11. write the compositions of common antacids, Gelusil and Diovol.
Ans. Gelusil is composed of aluminium hydroxide, magnesium trisilicate and siloxane.
Diovol is composed of aluminium hydroxide, magnesium carbonate and magnesium hydroxide.
12. What are systemic and non-systemic antacids? Give examples of each.
Ans. Systemic antacids: The antacids which get readily dissolved and absorbed in blood and hence, disturb the acid-base balance of the body are called systemic antacids. They follow a definite mechanism to reduce acidity. Common examples include sodium bicarbonate, sodium citrate etc.
Non-systemic antacids: Non-systemic antacids are insoluble and are very poorly absorbed in the blood. Hence, they do not disturb the acid-base balance of the body. They do not follow any definite mechanism of functioning as antacids. Some common examples include aluminium hydroxide, different calcium salts etc.
13. What is milk of magnesia? Write its uses and side effects.
Ans. Milk of magnesia: Aqueous suspension of magnesium hydroxide is known as milk of magnesia. It contains 7-8% magnesium hydroxide.
Uses: (1) Magnesium hydroxide is an effective antacid. It rapidly reacts with HCl produced in stomach and neutralises the acid. Hence, it provides quick relief from acidity. (2) It is often used along with Al(OH)3 as antacid. This provides quick relief from acidity and eliminates any possibility of constipation due to Al(OH)3.
Side effects: (1) HCl reacts with Mg(OH)2 to produce MgCl2 which may cause diarrhoea. (2) Deposition of magnesium in the kidneys can cause toxic effect in the body.
14. Discuss the uses and side effects of e effects of sodium bicarbonate as antacid.
Ans. Uses: Sodium bicarbonate is highly soluble in water and hence, it works rapidly as an antacid. However, it acts for a relatively short duration.
Side effects: (1) Sodium bicarbonate produces CO2 in the stomach which may cause uneasiness and infection in the bile duct. (2) It disturbs the acid-base balance of the body. (3) It increases the Na+ ion concentration in blood which may cause high blood pressure.
15. Discuss the uses and side effects of aluminium hydroxide as antacid.
Ans. Uses: Aluminium hydroxide is used to decrease the hyperacidity of stomach. It works slowly and hence, takes time to relieve the pain. Large amount of acid is produced in the stomach due to peptic ulcer, dyspepsia etc. These result in chest pain, uneasiness, acidity etc. Aluminium hydroxide readily reacts with the excess acid in the stomach and provides relief.
Side effects: (1) Excess use of Al(OH)3 may cause constipation. (2) Prolonged and continuous use of Al(OH)3 may decrease the phosphate level of blood. As a result, excretion of Cu through urine increases which results in renal rickets.
16. State whether the aqueous solution of all normal salts are neutral in nature.
Ans. A normal salt is produced due to complete neutralisation of an acid and a base. So, the aqueous solution of a normal salt should be neutral in nature. However, there are a number of normal salts whose aqueous solutions are either acidic or alkaline in nature. The cations and anions produced from these salts in their aqueous solutions react with water molecules thereby increasing the concentration of H3O+ or OH– ions. As a result, the solution becomes acidic or alkaline accordingly. Apart from a salt of strong acid and strong base, the solutions of all other normal salts are either acidic or alkaline.
For example, NH4Cl is a normal salt of strong acid, HCl and weak alkali, NH4OH. The hydrolysis of this salt produces excess H3O+ ions in water and the aqueous solution becomes acidic.
On the other hand, Na2CO3 is a salt of weak acid, H2CO3 and strong base, NaOH. It hydrolyses to produce excess OH– ions in the solution thus, making it alkaline in nature.
17. Baking soda was used in earlier days as antacid. But now-a-days Mg(OH)2, Al(OH)3 are used instead. State the reason behind it.
Ans. Baking soda can disturb the acid-base equilibrium of the body is it can be absorbed by blood. Baking soda can also interfere with how the body absorbs some medications.
Now-a-days, Mg(OH)2 and Al(OH)3 are used instead. These antacids do not destroy the acidbase equilibrium inside the body as they do not get absorbed by blood. These antacids form corresponding salt and water to decrease the acidity by neutralising the excess amount of HCl present in stomach.
18. What is meant by basicity of an acid?
Ans. The number of replaceable hydrogen atoms present in 1 molecule of an acid or the number of H+ (or H3O+) ions furnished by 1 molecule of the acid in aqueous solution is called the basicity of the acid. For example, 1 molecule of HCl dissociates in aqueous solution to produce one H+ ion while 1 molecule of H2SO4 dissociates to give two H+ ions. So, the basicity of HCl and H2SO4 are 1 and 2 respectively.
19. What is meant by acidity of a of a base?
Ans. The number of OH- ions produced by 1 molecule of a base is called the acidity of the base. In other words, it may be defined as the number of molecules of a monobasic acid required to neutralise 1 molecule of the base quantitatively. For example, 1 molecule of NaOH furnishes one OH– ion in aqueous solution and 1 molecule of monobasic acid, HCl is required for complete neutralisation of 1 molecule of NaOH. Hence, acidity of NaOH is 1.
20. Define basic salts with examples.
Ans. The salt produced when the replaceable hydroxyl radicals of a base or an alkali are partially replaced by an acid radical is known as basic salt. Some examples of basic salts are basic lead nitrate [Pb(OH)NO3], basic lead chloride [Pb(OH)Cl] etc.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. An example of an acid-base indicator is
A. methyl alcohol
B. methyl orange
C. methyl chloride
D. methylamine
Ans. B
2. In neutral solution, the colour of litmus is
A. violet
B. red
C. orange
D. yellow
Ans. A
3. An indicator which shows pink colour in alkaline solution is
A. litmus
B. methyl orange
C. phenolphthalein
D. methyl red
Ans. C
4. In neutral solution, the colour of methyl red indicator is
A. red
B. blue
C. yellow
D. orange
Ans. D
5. In acidic solution, the colour of litmus is
A. blue
B. red
C. yellow
D. violet
Ans. B
6. An aqueous solution of sodium carbonate (Na2CO3) is alkaline because it is a salt consisting of
A. strong acid and strong base
B. weak acid and weak base
C. strong acid and weak base
D. weak acid and strong base
Ans. D
7. An acid salt is produced when sodium hydroxide (NaOH) reacts with
A. HCl
B. HNO3
C. H2SO4
D. CH3COOH
Ans. C
8. The indicator used in the titration of a strong acid and a weak base is
A. methyl orange
B. litmus
C. phenolphthalein
D. any indicator
Ans. A
9. The indicator which is used in the titration of a weak acid and a strong base is
A. methyl orange
B. litmus
C. phenolphthalein
D. any indicator
Ans. C
10. The indicator which is used to differentiate between aqueous solutions of Na2CO3 and NH2Cl is
A. methyl orange
B. litmus
C. phenolphthalein
D. any indicator
Ans. C
11. The indicator used in the titration of a strong acid and a strong base is
A. methyl orange
B. methyl red
C. phenolphthalein
D. any indicator
Ans. D
12. The indicator suitable for titration of a weak acid and a weak base is
A. methyl orange
B. phenolphthalein
C. any indicator
D. no indicator is suitable
Ans. D
13. Omeprazole is used as a/an
A. pain killer
B. antipyretic
C. drug to decrease acid secretion in the stomach
D. analgesic
Ans. C
14. An example of a systemic antacid is
A. NaHCO3
B. Al(OH)3
C. NaOH
D. Ca(OH)2
Ans. A
15. An example of a non-systemic antacid is
A. NaHCO3
B. Al(OH)3
C. NaOH
D. Ca(OH)2
Ans. B
16. An example of a normal salt is
A. Na2HPO4
B. NaH2PO4
C. NaH2PO3
D. Na2SO4
Ans. D
17. Which of the following is the major component of milk of magnesia?
A. Fe(OH)3
B. Mn(OH)2
C. Mg(OH)2
D. Ca(OH)2
Ans. C
Answer in brief
1. Name two acid-base indicators.
Ans. Methyl orange and phenolphthalein are two widely used acid-base indicators.
2. How do indicators indicate the end point of an acid-base neutralisation reaction?
Ans. An indicator indicates the end point of an acid-base neutralisation reaction by changing its colour.
3. Name an indicator which exhibits orange colour in neutral solution.
Ans. Methyl orange.
4. Name two indicators which exhibit yellow colour in alkaline solution.
Ans. Methyl orange and methyl red exhibit yellow colour in alkaline solution.
5. Give an example of an acid which can produce two types of salts by reacting with a base.
Ans. Sulphuric acid (H2SO4) reacts with a base to produce two types of salts (acid salt and normal salt). It partially reacts with NaOH to produce an acid salt, sodium bisulphate (NaHSO4) and on complete neutralisation it produces a normal salt, sodium sulphate (Na2SO4).
6. Name an indicator that can be used for the neutralisation reaction of CH3COOH and NaOH.
Ans. Phenolphthalein.
7. Name an indicator that can be used for the neutralisation reaction of H2SO4 and NaOH.
Ans. Any indicator.
8. Name an indicator that can be used for the neutralisation reaction of CH3COOH and NH4OH.
Ans. No indicator is suitable for the neutralisation reaction of weak acid, CH3COOH and weak base, NH4OH.
9. What will be the colour of the Na2CO3 solution on addition of methyl orange?
Ans. Yellow.
10. Neutralisation reaction is mainly the reaction of which ions?
Ans. H⊕ and OHΘ.
11. State the number of molecules of caustic soda required to neutralise 1 molecule of sulfuric acid.
Ans. 2 molecules of caustic soda are required.
12. What is the chemical name of widely used antacid, Zantac?
Ans. The chemical name of widely used antacid Zantac is ranitidine.
13. How does the stomach get affected if acidity continues for a prolonged period of time?
Ans. If acidity continues for a prolonged period of time, it causes ulcer in the stomach.
14. Name the acid salts produced when orthophosphoric acid reacts with NaOH.
Ans. Disodium hydrogen phosphate (Na2HPO4) and sodium dihydrogen phosphate (NaH2PO4).
15. Name an acid which always forms normal salt when it reacts with bases.
Ans. Hydrochloric acid (HCl).
16. Give the name and formula of an acid salt which is formed due to partial neutralisation of a monobasic acid.
Ans. An acid salt which is formed due to partial neutralisation of a monobasic acid such as, hydrogen fluoride (HF) is potassium bifluoride (KHF2).
17. What is the reason behind ‘alkalosis disease’?
Ans. Alkalosis disease can be caused by taking excess NaHCO3 as antacid.
18. Give an example of a salt without a metal atom.
Ans. Ammonium chloride (NH4Cl).
19. State about the nature of the aqueous solution of NaHSO4.
Ans. The aqueous solution of NaHSO4 is acidic.
20. Write down the names of two main components of antacid.
Ans. Aluminium hydroxide [Al(OH)3] and magnesium hydroxide [Mg(OH)2].
21. Write down the formula of the acid salt obtained from the reaction of phosphoric acid and magnesium hydroxide.
Ans. MgHPO4,(magnesium hydrogen phosphate).
22. What is ‘milk of magnesia’?
Ans. Aqueous suspension of magnesium hydroxide.
Fill in the blanks
1. The indicator which turns colourless in acidic solution is ………….
Ans. phenolphthalein
2. The colours of methyl orange and methyl red change in …………. solution.
Ans. acidic
3. …………. indicator turns blue in alkaline medium.
Ans. Litmus
4. Universal indicator is actually a …………. of some selective indicators.
Ans. mixture
5. ………… types of salts are produced when NaOH reacts with H2SO4.
Ans. Two
6. HNO3 reacts with NaOH to produce ……….. type of salt.
Ans. one
7. The name of the salt, CuSO4 · Cu(OH)2 is …………
Ans. basic copper sulphate
8. ………. and ……….. are produced in an acid-base neutralisation reaction.
Ans. Salt, water
9. The indicators used in acid-base titrations are generally very weak organic ………. or organic ………
Ans. acids, bases
10. …………. is an example of a systemic antacid.
Ans. Sodium bicarbonate
11. ……….. is an example of a non-systemic antacid.
Ans. Aluminium hydroxide
State whether true or false
1. Methyl orange indicator turns colourless in basic solution.
Ans. False
2. Phenolphthalein can be used as an indicator in the neutralisation reaction of CH3COOH and NH4OH.
Ans. False
3. Sodium chloride and zinc sulphate belong to the category of normal salt.
Ans. True
4. Excessive use of aluminium hydroxide as antacid for a long period of time may cause renal rickets.
Ans. True
5. Excessive use of chemical fertilisers on agricultural lands for a long period of time makes the soil alkaline.
Ans. False
6. Sodium carbonate is an alkali.
Ans. False
7. Oxalic acid is a dibasic acid.
Ans. True
8. Sodium sulfate is an acid salt.
Ans. False