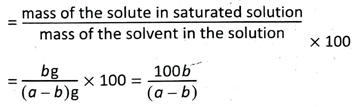WBBSE 9th Class Science Solutions Physical Science & Environment Chapter – 4.3 Solution
West Bengal Board 9th Class Science Solutions Physical Science & Environment Chapter – 4.3 Solution
WBBSE 9th Class Physical Science & Environment Solutions
Synopsis
- A solution is a homogeneous mixture of two or more substances in which the amount of each constituent can be varied within a certain limit.
- The component of a solution which is present in greater amount is called the solvent. The solution has the same physical state as that of the solvent. On the other hand, the component of the solution which is present in lesser amount is called the solute.
- A colloidal solution is a stable heterogeneous system of two immiscible phases in which one phase (solid, liquid or gas) is dispersed as particles with diameter ranging from 10-7-10-5 cm, in another phase (solid, liquid or gas).
- Colloid is not a special type of substance, rather it is a state of the substance.
- The medium in which the colloidal particles remain uniformly dispersed is known as the dispersion medium and the substance whose particles remain dispersed in a colloidal solution is called the dispersed phase.
- When light rays are passed through a colloidal solution, the rays get scattered by the colloidal particles and as a result the path of light becomes clearly visible. This phenomenon is known as Tyndall effect.
- Tyndall effect helps to distinguish between true solutions and colloidal solutions.
- On the basis of the physical states of the dispersion medium and dispersed phase, colloids are classified into eight groups. These are-sol, solid sol, gel, emulsion, solid aerosol, liquid aerosol, solid foam and foam.
- An emulsion is a colloidal solution in which both the dispersed phase and dispersion medium are liquid.
- Emulsions are of two types: oil-in-water type and water-in-oil type emulsion.
- The chemical substances used to enhance the stability of an emulsion are called emulsifiers. Soaps and detergents are well known emulsifiers.
- A suspension is a heterogeneous and unstable system in which particles having diameter greater than 10-5 cm remain dispersed in the solvent. On standing, the particles slowly separate out from the mixture and settle at the bottom.
TOPIC – A
True Solution, Colloid and Suspension, Dissolution of Smaller lon or Molecules and Larger Molecules in Water
SHORT AND LONG ANSWER TYPE QUESTIONS
1. What is a solution?
Ans. If a homogeneous mixture of two or more substances (solid, liquid or gas) have uniform properties (in terms of constituents and structure) throughout the mixture and the amounts of the constituents can be varied within certain limits, then the homogeneous mixture is said to be a solution.
2. What do you mean by solvent and solute?
Ans. Solvent: The component of a solution which is generally present in greater amount and whose physical state is the same as that of the solution is known as the solvent.
Solute: The component of a solution which is present in lesser amount and which remains dissolved in another solid, liquid or gaseous substance to form a homogeneous mixture (solution) is called a solute. For example, sugar dissolves in water to form a homogeneous mixture. Here, sugar is the solute.
In some solutions, where both solvent and solute are in the same phase, the terms solvent and solute is defined with respect to their relative quantities in the solution. For example, when 70 parts of alcohol mix with 30 parts of water, alcohol is considered as the solvent and water as the solute. On the other hand, in a mixture of 70 parts of water and 30 parts of alcohol, water is the solvent and alcohol is the solute.
3. All solutions are mixtures but all mixtures are not solutions. Justify.
Ans. If a mixture of two or more substances is homogeneous in nature and their composition . can be varied only within a certain limit, then the mixture is called a solution. For example, common salt dissolved in water forms a solution.
However, a mixture may also be heterogeneous in nature, in which the composition is not uniform throughout and can be changed in any proportion. This type of mixtures cannot be termed as solution. For example, a mixture of sand and sugar cannot be called a solution.
Thus, all solutions are mixtures but all mixtures are not solutions.
4. Define homogeneous and heterogeneous mixtures.
Ans. Homogeneous mixtures: A homogeneous mixture is a solid, liquid or gaseous mixture that has the same proportions of its components throughout any given sample. E.g., air or sugar solution.
Heterogeneous mixture: A heterogeneous mixture is simply a mixture that is not uniform in composition. It has components in which proportions vary throughout the sample. E.g., mixture of sand and sulphur powder.
5. Why is an aqueous solution of sugar us solution of sugar called a true solution?
Ans. When sugar is added to water, the sugar molecules occupy the intermolecular spaces between the water molecules and dissolve in it. As a result, the sugar molecules form a homogeneous mixture with water and cannot be separated easily. This is why an aqueous solution of sugar is called a true solution.
6. Discuss the properties of a true solution.
Ans. A true solution possesses the following properties-
- A true solution is a homogeneous mixture of solvent and solute. It has uniform properties (in terms of components, physical properties and structural features) throughout the solution.
- The solute particles present in a true solution are so small (particle diameter is less than or equal to 10-8 cm) that they cannot be seen even under an ultramicroscope.
- In a true solution, the solvent and solute may exhibit some changes in their physical properties, but their chemical properties remain unchanged.
- The relative amount of the components in a solution can be increased or decreased within a certain limit.
- The solute and solvent in a true solution cannot be separated by filtration or by gravitational separation. Even if the solution is kept undisturbed for a long period of time, the solute does not settle down.
- The components of a true solution can be separated by physical methods such as, evaporation, distillation or crystallisation.
- Heat may or may not be evolved or absorbed during formation of a true solution.
7. What is a colloidal solution?
Ans. A colloidal solution is said to be a stable heterogeneous system of two immiscible phases in which one phase (solid, liquid or gas) comprises of the particles with diameter ranging from 10-7 – 10-5 cm, is dispersed into another phase (solid, liquid or gas). For example, freshly precipitated ferric hydroxide when shaken with water and small amount of ferric chloride, it forms a colloidal solution.
8. What is meant by dispersion medium and dispersed phase of a colloidal solution?
Ans. Dispersion medium: The medium in which the colloidal particles remain uniformly dispersed is called the dispersion medium.
Dispersed phase: The component of a colloidal solution which remain uniformly dispersed in the dispersion medium and consists of particles with diameter ranging from 10-7 – 10-5 cm is called the dispersed phase.
Example: In a gold sol, water is the dispersion medium while gold particles form the dispersed phase.
9. State whether colloidal particles can be separated from a solution by filtration using a filter paper.
Ans. The pores of the filter paper are generally larger than 10-5 cm. So, the colloidal particles having diameter of 10-7 to 10-5 cm can easily pass through a filter paper. So, colloidal particles cannot be separated from a solution using a filter paper. On the other hand, colloidal particles cannot pass through a parchment paper. So, a parchment paper is effective in separating colloidal particles from a solution.
10. State the important properties of a properties of a colloidal solution.
Ans. A colloidal solution has the following properties-
- Heterogeneity: Colloidal solutions are heterogeneous in nature. The dispersed phase neither dissolves completely in the dispersion medium nor does it separate out from the dispersion medium. The colloidal particles remain dispersed in the dispersion medium.
- Tyndall effect: When a beam of light is allowed to pass through a colloidal solution, scattering of light by the colloidal particles occurs and the path of the beam through the solution gets illuminated and clearly visible. This phenomenon is called Tyndall effect.
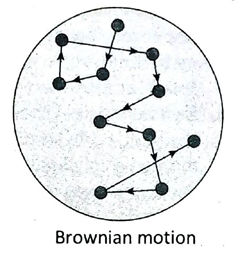
- Brownian motion: The colloidal particles in the dispersion medium are in continuous random motion moving in zigzag paths. This motion is known as Brownian motion.
- Electrophoresis: The colloidal particles are charged particles. If electricity is passed through a colloidal solution, then the colloidal particles move towards the oppositely charged electrodes. This movement of colloidal particles towards a specific electrode, under the influence of an electric field is called electrophoresis.
- Passage through filter paper and parchment paper: Colloidal particles can pass through filter paper but cannot pass through parchment paper.
11. What are the diameters of the solute particles in true solutions, colloidal solutions and suspensions?
Ans. In true solutions, diameter of solute particles is less than or equal to 10-8 cm or 0.1 nm.
In colloidal solutions, the diameter of solute particles ranges from 10-7 – 10-5 cm or 1-100 nm. In suspensions, the diameter of solute particles is greater than 10-5 cm or 100 nm.
Lyophilic and lyophobic sol: If the dispersed phase of a sol has high affinity for the molecules of dispersion medium, then it is called a lyophilic sol. Sols of starch, gum, gelatin, glue etc. are examples of such sol. If the dispersion medium of such sols is water, then these are called hydrophilic sols.
If the dispersed phase of a sol has a very little or almost no affinity for the molecules of dispersion medium, then it is called a lyophobic sol. Some common examples are ferric hydroxide [Fe(OH)3] sol, arsenius sulphide [As2S3] sol, etc. If water is the dispersion medium, then such sols are called hydrophobic sols.
12. Discuss the significance of Brownian motion.
Ans. Significance of Brownian motion are as follows-
- Brownian motion is an inherent evidence of the incessant motion of particles in a solution.
- The value of Avogadro’s number can be determined by measuring Brownian motion.
- Due to Brownian motion, the colloidal particles are in continuous motion. This motion prevents the particles to settle down even under gravitational force. Thus, Brownian motion plays a very significant role in stabilising a colloidal solution.
13. What is a suspension?
Ans. A heterogeneous and unstable system in which particles of a substance (usually a solid) of diameter greater than 10-5 cm remain suspended in another substance (usually a liquid) is called a suspension. On standing, the particles slowly separate out from the mixture and settle down at the bottom of the vessel.
Example: When finely powdered barium sulphate (BaSO4) is shaken vigorously with water, it is observed that the particles get evenly distributed throughout the solvent and remain suspended in water to form a suspension. If the beaker is kept undisturbed for some time, then barium sulphate particles begin to settle down at the bottom of the beaker.
14. State the properties of a suspension.
Ans. A suspension has the following properties-
- A suspension is a heterogeneous mixture.
- When kept undisturbed for some time, the suspended particles gradually settle down under gravity.
- The solution is turbid and hence light cannot pass through a suspension. However, a suspension may sometimes exhibit the Tyndall effect.
- The suspended particles are visible under ordinary microscopes and sometimes even to the naked eyes.
- The suspended particles cannot pass through a parchment paper or a filter paper.
15. Colloidal particles do not settle down at the bottom of the beaker, but particles of suspensions do. Explain with reason.
Ans. Due to Brownian motion of the colloidal particles, they are always in a continuous random motion. The possibility of aggregation of colloidal particles is thus very low. So, colloidal particles do not settle down at the bottom of the beaker.
On the other hand, particles of a suspension do not exhibit Brownian motion. Hence, on standing, the particles come closer to each other and aggregate to form larger particles which eventually settle down under gravity.
16. Explain why turbid water collected from ponds or rivers do not become clear on prolong standing?
Ans. Mud particles acts as the colloid particles in the turbid water of ponds or rivers. These colloid particles can not be precipitated out naturally. That is why those water do not become clear on prolong standing.
17. Why do colloidal solutions formed by different methods exhibit different colours?
Ans. The colour of a colloidal solution depends on the wavelength of visible light scattered by the colloid particles. The scattering of light, on the other hand, depends on the size of colloidal particles in the solution. The size of colloidal particles formed is different for different methods. So, a colloidal solution exhibits different colours when formed by different methods.
18. What is a sol? Give example.
Ans. Colloidal solutions in which the dispersed phase is a solid while the dispersion medium is a liquid is called a sol.
Example: Gold sol, sulphur sol, arsenius sulphide sol etc. In each of these sols, gold, sulphur and arsenius sulphide particles respectively form the dispersed phase while water acts as the dispersion medium.
19. What is an aerosol? How are they categorised?
Ans. An aerosol is a colloidal system in which the dispersion medium is a gas or air.
If the dispersed phase in an aerosol is solid, then it is called solid aerosol such as, smoke, dust particles floating in air etc. On the other hand, if the dispersed phase of an aerosol is liquid, then it is called liquid aerosol such as, fog, cloud etc.
20. What are alcosol and hydrosol?
Ans. Colloids with alcohol as dispersion medium ans having solids as dispersed phase are termed as alcosols.
Again, colloids whose dispersed phases and dispersion medium are solid and water respectively are termed as hydrosol.
21. State some important applications of emulsions.
Ans. Some important applications of emulsions are given below-
- Milk, butter, margarine, vanishing cream, cold cream used in our everyday life are all emulsions.
- Cleansing action of soap is due to the emulsification of grease with water. Water and soap together forms a colloidal solution and removes grease along with dirt and dust from clothes by forming emulsion.
- A wide variety of medicines like cod liver oil, vitamin B-complex are oil-in-water type emulsions. These are easily adsorbed by our digestive system and provide quick relief.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. Which of the following is a true solution?
A. aqueous solution of sugar
B. cod liver oil
C. mixture of sand and water
D. milk
Ans. A
2. Which of the following is a colloidal solution?
A. aqueous solution of sugar
B. mixture of BaSO4 and water
C. mixture of sand and water
D. milk
Ans. D
3. Which of the following is an emulsion?
A. curd
B. milk
C. milk of magnesía
D. soda water
Ans. B
4. The solution in which scattering of light can be observed is
A. blood
B. mixture of sand and water
C. aqueous solution of copper sulphate
D. aqueous solution of sodium chloride
Ans. A
5. The correct order of stability is
A. suspension < colloidal solution < true solution
B. colloidal solution < true solution < suspension
C. true solution < colloidal solution < suspension
D. colloidal solution < suspension < true solution
Ans. A
6. For which of the following solutions, the solute particles can pass through parchment paper?
A. milk
B. cod liver oil
C. aqueous solution of sugar
D. sand mixed with water
Ans. C
7. Which of the following solutions is homogeneous in nature?
A. milk
B. cod liver oil
C. aqueous solution of sugar
D. mixture of sand and water
Ans. C
8. Which of the following acts as dialyzer in human body?
A. lungs
B. kidneys
C. liver
D. stomach
Ans. B
9. In which of the following, the dispersion medium is not a liquid?
A. fog
B. foam
C. sulphur sol
D. cream
Ans. A
10. Milk is a type of
A. gel
B. foam
C. oil-in-water type emulsion
D. water-in-oil type emulsion
Ans. C
11. An example of a solid sol is
A. metal alloy
B. gold sol
C. paneer
D. pumice stone
Ans. A
12. An example of gel is
A. milk of magnesia
B. paneer
C. cream
D. lather of soap
Ans. B
13. A colloidal solution of a liquid dispersed in another liquid is known as
A. sol
B. gel
C. foam
D. emulsion
Ans. D
14. The easiest way to identify a colloidal solution is by
A. observing Tyndall effect
B. observing Brownian motion
C. electrodialysis
D. none of the above
Ans. A
15. The molecules of which of the following substances will not occupy the intermolecular spaces between water molecules when it is dissolved in water?
A. ethanol
B. sugar
C. glycerin
D. protein
Ans. D
16 In presence of colloidal particles, light rays get
A. refracted
B. reflected
C. scattered
D. deviated
Ans. C
17. For which of the following dispersion medium is not liquid?
A. fog
B. lather
C. sulphur sol
D. cream
Ans. A
18. Example of liquid aerosol
A. cloud
B. smoke
C. milk
D. cream
Ans. A
19. For which of the following dispersed phase and dispersion medium are liquid and gas respectively?
A. emulsion
B. sol
C. gel
D. liquid Aerosol
Ans. D
20. Example of emulsifier
A. Arrowroot
B. Gelatine
C. Dettol
D. Cod liver oil
Ans. B
21. Which one is suspension?
A. milk
B. milk of magnesia
C. cake
D. shaving cream
Ans. D
22. Which one is not an example of emulsion?
A. butter
B. shampoo
C. cake
D. shaving cream
Ans. D
Answer in brief
1. The diameter of a particle ‘Q’ present in water is 80 nm. What is the nature of the solution?
Ans. Q will form a colloidal solution.
2. Name a colloidal solution which is used in our daily life.
Ans. Milk.
3. Mention the type of solutions formed when sulphur is separately dissolved in water and alcohol.
Ans. Sulphur remains dispersed in water to form a colloidal solution. On the other hand, it completely dissolves in alcohol to form a true solution.
4. Give some examples of solid sol.
Ans. Coloured glass, ruby glass (Au/glass), gemstones, metal alloys etc.
5. The particulate matter in air belongs to which type of colloid?
Ans. Solid aerosol.
6. What is meant by gel?
Ans. Gel is a colloidal solution in which a liquid is dispersed in a solid. So, the dispersion medium is solid and dispersed phase is liquid. Jelly, gelatin etc. are some examples of gel.
7. Give some examples of liquid aerosol.
Ans. Fog and cloud are some examples of liquid aerosol.
8. Give an example of a colloid in which the dispersion medium is solid and the dispersed phase is gas.
Ans. The colloids in which the dispersion medium is solid and the dispersed phase is gas are known as solid foams. Some common examples are, cake, pumice stone (air dispersed in silicate compounds) etc.
9. Give some examples of hydrophilic colloids.
Ans. Starch, gelatin, protein, cellulose, soap etc. are some examples of hydrophilic colloids.
10. Give some examples of hydrophobic colloids.
Ans. Silver sol, gold sol, arsenius sulphide sol, ferric hydroxide sol etc. are some examples of hydrophobic colloids.
11. Which substance acts as the emulsifying agent in milk?
Ans. In milk, casein (which is a protein) acts as the emulsifying agent.
12. What is the type of colloid in which both the dispersion medium and dispersed phase are liquid known as?
Ans. The colloids in which both the dispersion medium and dispersed phase are liquid are known as emulsions.
13. Name some covalent compounds which are soluble in water.
Ans. Some covalent compounds which are soluble in water are sugar, alcohol, HCI etc.
14. Name a water soluble organic compound.
Ans. Sugar is a water soluble organic compound.
15. Name an electrovalent compound which is insoluble in water.
Ans. An electrovalent compound which is insoluble in water is barium sulphate (BaSO4).
16. Give an example of a colloidal solution in which the colloidal particles are large molecules.
Ans. Starch remains dispersed in water to form a colloidal solution. Here, large starch molecules are present as colloidal particles.
17. What is the diameter of solute particles in a true solution?
Ans. ≤ 10-8 cm.
18. What is the diameter of solute particles in a colloidal solution?
Ans. 10-7 – 10-5 cm.
19. What is the diameter of solute particles in a suspension?
Ans. 10-5 cm.
20. Which method is used for the separation of crystalloids from colloids?
Ans. electrodialysis.
21. Name the random, continuous motion exhibited by colloidal particles in a colloidal solution.
Ans. Brownian motion.
22. Why gas mixtures cannot form colloidal systems?
Ans. Gas mixtures always form homogeneous mixtures irrespective of the ratio in which they are mixed. Thus, they cannot form colloidal systems.
23. The suspended particulate matters belong to which type of colloids?
Ans. SPM belong to the solid aerosol class of colloid.
24. By which property colloids and True solutions can be distinguished?
Ans. Tyndall Effect.
25. Name an emulsifier used in daily life.
Ans. Soap or detergent.
26. Name the process by which crystaloid can be separated from colloid using diffusion through semi-permeable membrane.
Ans. Dialysis.
27. Which solution is used with H2SO4 to prepare colloidal Sulphur?
Ans. Hypo solution or the dilute solution of Sodium thiosulphate.
28. Name two diseases caused due to solid aerosol.
Ans. Asthma and silicasis.
29. Which protective colloid is used in icecream?
Ans. Gelatine.
30. Given an example of a solution where salute and solvent both are solid.
Ans. Brass.
31. Give an example of a colloid which is an essential component of our body.
Ans. Blood (colloidal solution of Albumin).
32. Give an example of a colloidal system where finer particles of water are dispersed in fat.
Ans. Butter.
33. Mention the dispersion phase in O/W type Emulsion.
Ans. Oil.
34. Give an example of colloid causing air pollution.
Ans. Solid aerosol.
35. Why Gelatine is used to prepare ice-cream?
Ans. Gelatine is used to produce ice-cream to give the stability of the colloidal system (icecream).
Fill in the blanks
1. True solutions are ………. in nature.
Ans. homogeneous
2. Colloidal solutions are ………. in nature.
Ans. heterogeneous
3. Milk is a colloid in which ……….. is the dispersed phase and ……….. is the dispersion medium.
Ans. fat, water
4. ………. solutions show Tyndall effect.
Ans. Colloidal
5. The colloidal particles in a colloidal solution can be observed by using ………..
Ans. ultramicroscope
6. When sodium chloride is dissolved in water, it forms a ……….. solution.
Ans. true
7. When starch is dissolved in water, it forms a ……….. solution.
Ans. colloidal
8. A ……….. is a colloidal solution in which the dispersed phase is solid.
Ans. sol
9. A ……….. is a colloidal system in which the dispersed phase is liquid and the dispersion medium is solid.
Ans. gel
10. An example of solid foam is ………….
Ans. cake
11. A colloidal system is known as ………… if the dispersed phase is a gas and the dispersion medium is solid.
Ans. solid foam
State whether true or false
1. The component of a solution, which is present in a lesser amount is known as the solute.
Ans. True
2. The amount of CaCl2 present in 500 mL of 25% CaCl2 solution is 125 g.
Ans. True
3. The solute particles of a colloidal solution can pass through parchment paper.
Ans. False
4. The solute particles of a true solution are visible under an ultramicroscope.
Ans. False
5. A true solution does not exhibit Tyndall effect.
Ans. True
6. If the diameter of solute particles is 10-4 cm, the corresponding solution is a true solution.
Ans. False
7. Colloidal system whose dispersed phase and dispersion medium both are gaseous, does not exist.
Ans. True
8. Particles of a true solution can not be observed even with the help of a powerful microscope.
Ans. True
9. Example of water-in-oil type emulsion is butter.
Ans. True
10. Colloidal solution are homogeneous.
Ans. False
11. Colloidal solution are transparent.
Ans. False
TOPIC – B
Solubility, Strength of Solution and Its Unit
SHORT AND LONG ANSWER TYPE QUESTIONS
1. Why is it necessary to mention temperature in the definition of solubility?
Ans. The solubility of a substance in a given solvent varies with temperature. Hence, the amount of a particular solute required to saturate 100 g of a solvent will be different at different temperatures. Thus, it is necessary to mention temperature in the definition of solubility.
2. Why is it necessary to mention a definite amount of solvent in the definition of solubility?
Ans. At a particular temperature, the amount of solute required to make a saturated solution depends on the amount of solvent. If greater amount of solvent is taken, then it can dissolve more amount of solute whereas, lesser amount of solvent dissolves lesser amount of solute. Hence, we cannot determine the amount of solute required to make a saturated solution, if the amount of solvent is not specified. Thus, it is necessary to specify the amount of solvent in the definition of solubility.
3. What are the units used to express the strength of a solution? Comment on their dependence on temperature.
Ans. Different units used to express the concentration or strength of a solution are volume percentage (% W/V), moles per litre or molarity (mol. L-1) and gram per litre (g.L-1).
Although mass or number of moles of a substance is independent of temperature but, volume of a solution changes with temperature. As all the units are dependent on volume of the solution, these are also dependent on temperature.
4. What is meant by molarity or moles per litre strength of a solution? Give example.
Ans. At a given temperature, the number of grammoles of a solute dissolved in 1 litre (or 1000 mL) of a solution is known as the molarity or moles per litre strength of the solution.
Example: 5 gram-moles of HNO3 is dissolved in 1 litre aqueous solution. Hence, the molarity of the solution will be 5. The molar strength is expressed as mol. L-1 or (M).
5. What is meant by gram per litre strength of a solution? Give example.
Ans. The amount of solute in gram dissolved per litre of the solution is expressed in gram per litre unit. Example: If 5.0g of sodium chloride is dissolved in 1 L of solution, then the strength of the sodium chloride solution in gram per litre unit will be 5. The unit is expressed as g.L-1 .
6. What changes will be ol observed in the (W/V) concentration of a solution if the temperature of the solution is raised?
Ans. The following changes will be observed in the (W/V) concentration of a solution if the temperature of the solution is raised-
- With the increase in temperature, the volume of the solution increases. Hence, the ratio of mass of solute to the volume of solution decreases. As a result, concentration of the solution also decreases.
- If some amount of solvent vapourises due to increase in temperature, then the volume of solution also decreases. Hence, concentration of the solution also increases.
7. Why molarity depends on temperature?
Ans. We know molarity of solution
Now volume of the solution changes with temperature. Thus molarity of the solution also changes with temperature, although the number of g mol of the solute remains unaltered. Hence molarity depends on temperature.
8. If the amount of the dissolved solute in a gram of saturated solution is b gram, calculate the solubility of the solute at that temperature.
Ans. We know, solubility of a solute at t°C
9. What are the factors on which the solubility of a substance depends?
Ans. The solubility of a substance in a given solvent depends on the following factors-
- Nature of solute: Different solutes dissolve to various extents in a given solvent at a particular temperature.
- Temperature: For most solid substances, solubility in a given liquid increases with the rise in temperature. However, there are some gases whose solubility in a given liquid decreases with the rise in temperature.
- Atmospheric pressure: At a particular temperature, the solubility of a gas in a liquid generally increases with the increase in pressure above the liquid and vice-versa. This is why CO2 dissolved in water under very high pressure comes out as bubbles when a sodawater bottle is opened.
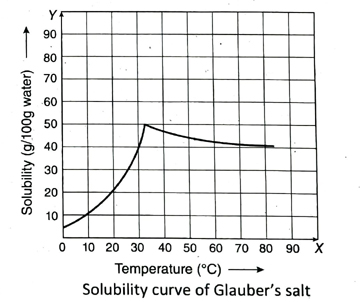
10. What is a solubility curve? Draw the solubility curve of Glauber’s salt in water and explain its solubility with the help of the In its solubility with the curve.
Ans. The curve obtained by plotting the solubility of a solute in a given solvent along Y-axis against temperature along X-axis, is called the solubility curve of that solute. This curve easily demonstrates the relation between temperature and solubility of a substance.
The solubility curve of Glauber’s salt in water (hydrated sodium sulphate) rapidly rises up with the rise in temperature upto 32.38°C. After 32.38°C, if the temperature is further increased, the solubility gradually starts decreasing. Above 32.38°C, hydrated crystals of sodium sulphate (Na2SO4 · 10H2O) give up ten water molecules to form anhydrous sodium sulphate (Na2SO4). So, above 32.38°C, the solubility curve actually represents the solubility of anhydrous sodium sulphate.
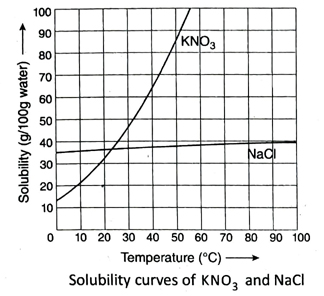
11. Draw the solubility curves of KNO3 and NaCl in water and mention the effect of temperature on solubility of these salts.
Ans. The solubility curve of KNO3 rises steeply with rise in temperature. This means that the solubility of KNO3 increases rapidly with the increase in temperature.
The solubility curve of NaCl is almost parallel to the X-axis. This indicates that the solubility of NaCl in water remains almost unchanged over a wide range of temperature.
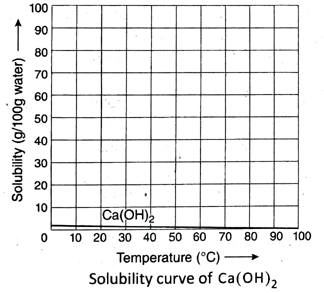
12. How does the solubility of Ca(OH)₂ in water changes with the rise in temperature? Explain with the help of its solubility curve.
Ans.
From the solubility curve, it can be said that the solubility of Ca(OH)2 in water decreases with the rise in temperature.
13. State the significance of solubility curve.
Ans.
- Without actually performing an experiment, the solubility of a substance at different temperatures can be determined with the help of the solubility curve.
- It helps to compare the solubilities of different substances at a given temperature.
- The solubility curve helps us to determine which substance will preferentially crystallise out if a solution of a mixture of substances is subjected to cooling or evaporation.
14. Discuss the effect of temperature on the solubility of a gas in a liquid.
Ans. In general, the solubility of a gas in a liquid decreases with the rise in temperature. For example, CO2 is soluble in water. At ordinary temperature, some amount of CO2 gas dissolves in water. However on heating, the dissolved CO2 fizzles out from the solution.
15. Discuss the effect of pressure on the essure on the solubility of a gas in a liquid.
Ans. At a particular temperature, the solubility of a gas in a liquid generally increases with the increase in pressure and decreases with the decrease in pressure. So, at a higher pressure relatively large amount of gas dissolves in a liquid. This principle is used in the preparation of soda water where excess CO2 is dissolved in water by applying pressure.
16. Why does soda water fizz when the cap of the bottle is removed?
Ans. At a particular temperature, the solubility of a gas in a liquid increases with increase in pressure. In soda water bottles, carbon dioxide is dissolved in water applying high pressure. When the cap of the bottle is removed, the pressure suddenly decreases and as a result the solubility of CO2 in water decreases. Consequently, excess carbon dioxide comes out from water and eventually causes the fizz.
17. A bottle containing liquor ammonia must be cooled before opening. Why?
Ans. A saturated aqueous solution of ammonia is called liquor ammonia. With rise in temperature, solubility of NH3 in water decreases and hence some NH3 gas comes out of the solution and causes excess pressure inside the bottle. If the bottle is opened under this condition, then excess NH3 gas along with some dissolved NH3 gas may spurt out from the bottle causing serious accident. However, if the bottle is cooled down, the excess NH3 again dissolves in water and no such accident occurs when the bottle is opened.
18. Why bubbles are formed when water is tied when heated?
Ans. Some amount of air generally remains dissolved in water at room temperature. Now solubility of gases in liquid decreases with increase in temperature. That is why solubility of air decreases when we heat the water and hence the excess air comes out of water forming bubbles.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. Water can dissolve ionic compounds, because water is a/an
A. covalent compound
B. ionic compound
C. polar covalent compound
D. liquid
Ans. C
2. The temperature upto which the solubility curve for the solubility of Glauber’s salt in water rises is
A. 25.5°C
B. 32.4°C
C. 35.2°C
D. 37.24°C
Ans. B
3. At a constant temperature, the solubility of a gas in a given volume of liquid is directly proportional to the pressure of the gas. This statement is known as
A. Boyle’s law
B. Charles’ law
C. Raoult’s law
D. Henry’s law
Ans. D
4. With rise in temperature, the solubility of potassium nitrate (KNO3) in water
A. decreases
B. increases
C. remains almost unchanged
D. initially increases and then decreases
Ans. A
5. Which of the following units of concentration is not affected by any change in temperature?
A. mass-volume percentage (% W/V)
B. mass percentage (% W/W)
C. moles per litre
D. gram per litre
Ans. B
6. With rise in temperature, the solubility of a gas in a liquid
A. increases
B. decreases
C. remains almost unchanged
D. initially increases and then decreases
Ans. B
7. With increase in temperature, the solubility of slaked lime in water
A. increases
B. decreases
C. remains same
D. increases firstly then decreases
Ans. B
8. Solubility does not depend upon
A. temperature
B. pressure
C. nature of solvent
D. gravitational force of the earth
Ans. D
9. With increase in pressure, the solubility of a solid in a liquid
A. increases
B. decreases
C. firstly increases and then decreases
D. remains same
Ans. D
Answer in brief
1. What is the unit of solubility?
Ans. Solubility is a unitless physical quantity. It has no unit.
2. Name some compounds whose solubility in water decreases with the increase in temperature.
Ans. The compounds whose solubility in water decreases with the increase in temperature are calcium sulphate (CaSO4), calcium nitrate [Ca(NO3)2], slaked lime or calcium hydroxide [Ca(OH)2], cerium sulphate [Ce2(SO4)3] etc.
3. Name a solid whose solubility in water remains almost constant with change in temperature.
Ans. Solubility of common salt or sodium chloride (NaCl) in water remains almost constant with change in temperature
4. Name a solid whose solubility in water initially increases with the rise in temperature but starts to decrease when the temperature is further increased.
Ans. The solubility of Glauber’s salt (Na2SO4 · 10H2O) in water initially increases with the rise in temperature up to 32.4°C and then the solubility begins to decrease when the temperature is further increased.
5. How is the solubility of a solid solute in a liquid solvent affected by pressure?
Ans. There is no effect of pressure on the solubility of a solid solute in a liquid solvent.
6. State Henry’s law.
Ans. At constant temperature, the solubility of a given gas in a given volume of liquid solvent is directly proportional to the pressure of that gas.
7. What will happen if a saturated solution of sugar prepared at room temperature is cooled down to 5°C ?
Ans. When a saturated solution of sugar prepared at room temperature is cooled down to 5°C, the solubility of sugar decreases and some amount of sugar settles down at the bottom.
8. How much NaCl is present in 50 mL of 20% aqueous solution of NaCl?
Ans. 50 mL of 20% aqueous solution of NaCl will contain 10 g of NaCl.
9. What change in W/V concentration of a solution will be observed with the rise in temperature?
Ans. If the solvent does not evaporate on heating, then the W/V concentration of a solution generally decreases if the temperature is increased.
10. Why does molarity of a solution depend on temperature?
Ans. Molarity of a solution is the number of moles of solute present per litre of a solution. As the volume of solution depends on temperature (although mass of solute is independent of temperature), the molarity of a solution also depends on temperature.
11. Why the statement-“Solubility of NaCO3 in water is 20″-is erroneous?
Ans. The temperature is not mentioned here which makes the statement erroneous.
12. What do you mean by the statement- “Solubility curve of NaCl is almost parallel to x -axis?
Ans. It means that solubility of NaCl in water remains almost unaltered with change in temperature.
13. State whether the solubility of gas in water increases or decreases with decrease in pressure.
Ans. Solubility of gas in water decreases with decrease in pressure.
14. Which of the following solutions of higher concentration: 100 g/L NaOH and 10 molar NaOH.
Ans. Concentration of NaOH in 10 molar NaOH solution is higher.
Fill in t Fill in the blanks
1. If 5g of a salt is dissolved in 50 mL water, then the strength of the solution will be ………. % (W/V).
Ans. 10
2. The solubility of those substances which absorb heat during dissolution in water, ………. with the rise in temperature.
Ans. increases
3. The chemical substance added to a mixture of two immiscible substances to increase the stability of the emulsion is called a/an ………. agent.
Ans. emulsifying
4. The solubility-curve for the solubility of NaCl in water is almost ………. to the temperature-axis.
Ans. parallel
5. The solubility curve of ………. rises up with the rise in temperature and then starts decreasing after reaching a maximum.
Ans. Glauber’s salt
6. The solubility of calcium hydroxide in water ………. with rise in temperature.
Ans. decreases
7. The solubility of Glauber’s salt at 32.4°C as obtained from its solubility curve actually represents the solubility of ……….
Ans. anhydrous Na2SO4
8. CO2 is dissolved in water at ………. pressure to produce soda water.
Ans. high
9. With rise in temperature, the % (W/V) strength of a solution ……….
Ans. decreases
10. To express the solubility of a substance, mentioning of ………. is necessary.
Ans. temperature
11. If 10 g of NaOH is dissolved in 100 ml of NaOH solution, strength of the solution is ……….
Ans. 10%
12. Solubility of CO2 in water increase in temperature. with
Ans. decreases
13. Solubility of solids in liquid depends on ……….
Ans. temperature
State whether true or false
1. The amount of CaCl2 present in 500 mL of 25% CaCl₂ solution is 125 g.
Ans. True
2. The unit of solubility is gram per litre.
Ans. False
3. The solubility of cerium sulphate decreases with the rise in temperature.
Ans. True
4. Molarity of a solution is independent of temperature.
Ans. False
5. The solubility of a gas in a liquid usually decreases with the increase in pressure above the liquid.
Ans. False
6. W/V concentration of a solution increases with the rise in temperature.
Ans. False
TOPIC – C
Saturated, Unsaturated, Supersaturated Solution
SHORT AND LONG ANSWER TYPE QUESTIONS
1. What is a saturated solution?
Ans. A solution in which, the maximum amount of solute remains dissolved in the solvent at a given temperature, is called a saturated solution. If more amount of solute is further added to the solution at that temperature, the excess solute settles at the bottom, but the concentration of the solution remains unchanged.
2. What is an unsaturated solution?
Ans. An unsaturated solution is one in which, at a particular temperature, the solvent has the capacity to dissolve more amount of solute. On adding more solute, the concentration of the solution increases and after some time the solution becomes saturated.
3. Differentiate between saturated and unsaturated solutions.
Ans. The differences between saturated and unsaturated solutions are as follows-
| Saturated solution |
Unsaturated solution |
| 1. At a given temperature, if excess solute is added to a saturated solution, it settles down at the bottom of the beaker instead of getting dissolved. |
1. At a given temperature, if excess solute is added to an unsaturated solution, then it partially or completely dissolves in the solvent. |
| 2. At a given temperature, if excess solute is added to a saturated solution, no change is observed in its density. |
2. At a given temperature, if excess solute is added to an unsaturated solution, its density increases. |
| 3. At a given temperature, if excess solute is added to a saturated solution, then the dissolved solute and undissolved solute remain in dynamic equilibrium with each other.. |
3. No such equilibrium is observed in case of unsaturated solutions. |
4. How will you convert a saturated solution into an unsaturated solution?
Ans. A saturated solution can be converted to an unsaturated solution by the following methods-
- At a particular temperature, a saturated solution can be made unsaturated by adding some more amount of solvent.
- In most cases, on heating, a saturated solution becomes unsaturated at a higher temperature.
5. How will you convert an unsaturated solution into a saturated solution?
Ans. An unsaturated solution can be converted to a saturated solution by the following methods –
- At a particular temperature, an unsaturated solution can be made saturated by adding some more amount of solute.
- In most cases, on cooling, an unsaturated solution becomes saturated at a lower temperature.
- Evaporating some amount of solvent from a solution by heating and then subsequently cooling the solution to its initial temperature will decrease the amount of solvent in the solution. The solution thus obtained is a saturated solution at that temperature.
6. Why the saturated solution of glauber salt cannot be unsaturated by heating the solution?
Ans. The solubility of glauber salt (Na2SO4 · 10H2O) increases rapidly with increase in temperature upto 32.4°C. At a higher temperature than 32.4°C, the salt looses its water of crystallisation and transform to anhydrous sodium sulphate. On increasing the temperature above 32.4°C solubility of anhydrous sodium sulphate decreases with increase in temperature. As a consequence, the excess salt gets precipitated out from the saturated solution. Hence the above solution cannot be unsaturated by heating.
7. What is a supersaturated solution?
Ans. A supersaturated solution at a particular temperature is defined as the solution in which the solvent contains more solute at a specific condition than that required to form a saturated solution at that temperature.
8. State two important characteristics of a supersaturated solution.
Ans. Two important characteristics of a supersaturated solution are-
- A supersaturated solution is highly unstable. Excess solute from the solution settles down at the bottom of the beaker on slightly shaking the solution, by adding a small crystal of the solute or in presence of dust particles.
- On adding more solute to a supersaturated solution at a particular temperature, the concentration of the solution decreases.
9. How will you prepare the super saturated solution of sodium thiosulphate?
Ans. A small amount of hypo or sodium thiosulphate crystal is taken in a test tube and closed at top with cotton. The test tube is then immersed partially in boiling water bath. Crystals lose their water of crystallisation and get dissolved in that water forming a clear solution. The test tube is then cooled, without stirring, to room temperature. The solution thus obtained is the super saturated solution of sodium thiosulphate.
10. Differentiate between saturated and supersaturated solutions.
Ans. The differences between saturated and supersaturated solutions are as follows-
| Saturated solution |
Supersaturated solution |
| 1. A solution in which, at a given temperature, the maximum amount of solute remains dissolved in the solvent is called a saturated solution. |
1. A supersaturated solution at a particular temperature is the solution in which the solvent contains more solute at a specific condition than that is required to form a saturated solution. |
| 2. Saturated solutions are stable in nature. |
2. Supersaturated solutions are unstable in nature. |
| 3. At a given temperature, if excess solute is added to a saturated solution, no change is observed in its density. |
3. At a given temperature, if excess solute is added to a supersaturated solution, its density decreases because some solute settles down at the bottom of the beaker. |
11. with the help of a simple experiment, how will you determine whether a solution is saturated, unsaturated or supersaturated?
Ans. A solution of a given solute in a given solvent is taken. Some more amount of the solute is added to the solution. The following observations will help us to identify whether this solution is saturated, unsaturated or supersaturated-
- If the solute completely or partially dissolves in the solution on stirring at the same temperature, then the solution is an unsaturated solution.
- If the added solute deposits at the bottom of the beaker instead of dissolving and its concentration remains unchanged, then it is a saturated solution.
- If on addition of excess solute, the added solute separates out from the solution and settles at the bottom of the beaker instead of dissolving and the amount of deposited solute is more than the added amount of solute, then the solution is a supersaturated solution.
12. You have been given a saturated solution of common salt at room temperature. If the temperature of the solution has been reduced to 5°C by keeping it in an ice block, will the solution will the solution remain saturated? What will be the probable observations?
Ans. The solution will remain saturated.
Solubility of common salt changes slightly with temperature. So by cooling the solution only a little amount of salt will be observed to get precipitated out.
13. What is the reason behind the damage of health of the paint workers?
Ans. Many volatile substances like methylated spirit, tarpene oil, naptha, acetone etc. are used as solvent of paints during painting. These solvents evaporated at room temperature and enter easily to the body of the paint workers through inhalation. These can easily be in contact with the skin of the workers also.
These solvents cause allergy and several bronchiole or lung diseases which causes harm to the health of the workers involved in painting.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. A supersaturated solution in water can be prepared by
A. CuSO4 · 5H2O
B. Na2SO4 · 10H2O
C. FeSO4 · 7H2O
D. Na2S2O3 · 5H2O
Ans. D
2. The amount of dissolved solute present in a supersaturated solution compared to the maximum amount of solute that can be present in a saturated solution at a given temperature is
A. less
B. more
C. equal
D. none of these
Ans. B
3. Formula of hypo
A. Na2SO4 · 10H2O
B. Na2S2O3
C. Na2S2O3 · 5H2O
D. NaHSO4
Ans. C
4. What happens when the temperature of the KNO3 solution saturated at 80°C reduces to 40°C?
A. no change
B. solution will become unsaturated
C. some amount of KNO3 will settle down
D. solution will become supersaturated
Ans. C
5. The solution in which more solute can be dissolved, is
A. saturated solution
B. unsaturated solution
C. supersaturated solution
D. colloid
Ans. B
6. If some amount of solute is added to a saturated solution at a definite temperature, density of the solution will
A. increase
B. reduce
C. remain same
D. none of the above
Ans. C
Answer in brief
1. Classify solutions on the basis of their concentration.
Ans. On the basis of their concentration, solutions can be classified into three types-saturated solution, unsaturated solution and supersaturated solution.
2. Among saturated, unsaturated and supersaturated solutions, which one is the least stable?
Ans. A supersaturated solution is the least stable.
3. Name a solid which can form a supersaturated solution.
Ans. Sodium thiosulphate pentahydrate (Na2S2O3 · 5H2O), commonly known as hypo, can form a supersaturated solution.
4. State whether the solubility of common salt dissolved in a cup of water will be equal in Kolkata and Sikkim.
Ans. The solubility of a substance salt depends on temperature. As the temperature of the two places are different, the solubility of common salt in a cup of water will also be different.
5. What amount of of acid (in g) is present per litre of 2 (M) H2SO4 solution? (Molecular mass of H2SO4 is 98)
Ans. Per litre of 2 (M) H2SO4 solution will contain 98 × 2 = 196 g of acid.
6. Which type of solutions cannot form a saturated solution?
Ans. Solutions consisting of two miscible liquids can never form a saturated solution. For example, a solution of water and ethyl alcohol cannot form a saturated solution.
7. On cooling a saturated solution, whether it remains saturated or not?
Ans. If we cool a saturated solution, some amount of solute gets precipitated out. But the remaining solution remains saturated.
Fill in the blanks
1. A ………….. solution is one, whose concentration increases if some more solute is added to the solution.
Ans. unsaturated
2. In a ………… solution, the concentration remains unchanged even if some amount of solute is added to it.
Ans. saturated
3. A saturated solution dissolves more solute under a specific condition to produce ……….. solution.
Ans. supersaturated
4. At a particular temperature, the dissolved solute particles in a saturated solution is in ………… with the precipitated particles.
Ans. equilibrium
5. At a given temperature, if some more solute is added to a supersaturated solution, its solubility …………..
Ans. decreases
6. Unsaturated solution can absorb more …………
Ans. solute
State whether true or false
1. An unsaturated aqueous solution of ammonia is called liquid ammonia.
Ans. False
2 Supersaturated solutions are stable in nature.
Ans. False
3. A saturated solution remains saturated at any temperature.
Ans. False
4. When more solute is added to a saturated solution, the excess solute will be sedimented.
Ans. True
5. Supersaturated solution cannot be prepared by heating copper sulphate crystal in waterbath.
Ans. True
TOPIC – D
Crystallisation, Motion of Particles in Solution and Different Solvents Other Than Water
SHORT AND LONG ANSWER TYPE QUESTIONS
1. What are crystals? On which factors does the shape of a crystal depend?
Ans. Homogeneous solid substances having definite geometrical shape and bounded by symmetrically arranged plane surfaces that meet up at sharp edges are known as crystals. The substances formed of crystalline particles are known as crystalline substances. Some common examples are, alum, diamond, sugar, copper sulphate etc. The shape of a crystal entirely depends on the number of particles (atoms, molecules or ions) present in the crystal and their geometrical arrangement.
2. What do you mean by crystallisation and residue?
Ans. Crystallisation: The process by which crystals of a substance are formed from its saturated solution or molten state either by cooling the solution or by sublimation is known as crystallisation.
Residue: The remaining solution that is left behind in the beaker after a solid is separated out as crystals from its saturated solution on cooling is called residue.
3. What is the primary condition for the purification of a solid by crystallisation?
Ans. To purify a solid by crystallisation method, the solid must be soluble in a specific solvent while the impurities present in the solid must be insoluble or have different solubilities in that particular solvent.
4. Mention two important applications of crystallisation.
Ans. (1) Sea water contains different impurities along with dissolved salts. The method of crystallisation is applied for the removal of those impurities and extraction of salt from sea water.
(2) Impure samples of potash alum [K2SO4 · Al2(SO4)3 · 24H2O] and nitre (KNO3) are purified by crystallisation.
5. Crystallisation is a more effective method for purifying a solid than evaporation. Explain.
Ans. Crystallisation is a more effective method for purifying a solid than evaporation because of the following reasons
- During evaporation, the solvent is evaporated from the solution by applying heat. However on heating, some solids may decompose to produce new substances or substances like sugar may also get burnt to form a black mass of carbon particles.
- During evaporation, the solid impurities present in the solution do not separate out from the solution. So, the residue may contain large amount of impurities along with the desired substance. Thus, the solid obtained by evaporation may not be pure. However, during crystallisation, the impurities remain in the solution, while the solid crystallises out in pure form.
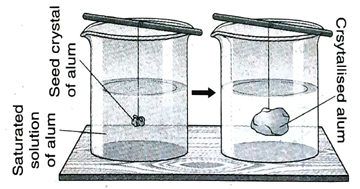
6. What is overgrowth? What is seed crystal? Give examples.
Ans. When a small crystal of the solute is dipped into its saturated solution with the help of a thread, the crystal grows bigger in size due to deposition of more solute crystals on its surface. This phenomenon is known as overgrowth of crystal.
Seed crystal is a small crystal of the solute, dipped into its saturated solution with the help of a thread. The crystal becomes larger due to deposition of more solute crystals on its surface.
If a small crystal of potash alum is dipped into a saturated solution of alum, alum starts depositing on the surface of the crystal and it grows bigger in size. Here, the small crystal is the seed crystal and the phenomenon is called overgrowth.
7. Mention some uses of seed crystals in different industries.
Ans. Seed crystals find important applications in many industries, such as-
- During production of cane sugar (sucrose) from sugarcane juice, pure crystals of sugar are used as seed crystals.
- Seed crystals are used to accelerate the crystallisation of precious stones (like diamond), gemstones (like sapphire) and semiconductors (like silicone, germanium etc.)
- In Baeyer’s process of extraction of alumina, hydrated crystals of Al2O3 are used as seed crystals to accelerate precipitation of alumina.
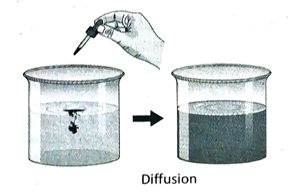
8. What is diffusion?
Ans. The phenomenon of spontaneous mixing of two or more substances irrespective of their nature and molar mass, without any external help to form a homogeneous mixture is called diffusion. In a solution, the solute particles move from a region of higher concentration to a region of lower concentration due to diffusion until a homogeneous mixture is formed.
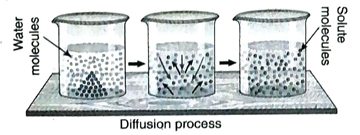
9. How does diffusion take place?
Ans. In a solution, both solvent and solute molecules move incessantly due to their high kinetic energy.
Consequently, the solute molecules collide among themselves as well as with the solvent molecules leading to continuous and random motion (Brownian motion). As a result, the solute particles move from a region of higher concentration to a region of lower concentration by the process of diffusion until an equilibrium is reached.
10. “Like dissolves like”- explain the explain the statement with examples.
Ans. When a solute is added to a solvent, three different types of interactions take place in the solution- (1) solvent-solventinteraction, (2) solute-solute interaction and (3) solvent-solute interaction.
When the nature and extent of these three interactions are similar, then the solute dissolves in the solvent. For example, polar solutes like sodium chloride dissolve easily in polar solvents like water. In this solution, the interaction between two water molecules is almost identical to the interaction between sodium chloride molecules as well as that between water and sodium chloride molecules. Similarly, a non-polar solute is found to be highly soluble in non-polar organic solvents.
11. Write some uses of methyl alcohol as a some uses of methyl alcohol solvent.
In different industries, methyl alcohol is extensively used as a solvent for colours, varnishes, celluloid substances, cements, fats etc.
12. Write some uses of ethyl alcohol as a me uses of ethyl alcohol as a solvent.
Ans. Ethyl alcohol is a very important solvent. It is used in different industries to dissolve resins, soaps, varnishes, colours, rayons, lax, synthetic rubbers, synthetic fibres, dyes etc. Ethyl alcohol is also used extensively as a solvent in pharmaceutical industries.
13. Write some uses of acetone as a solvent.
Ans. Acetone is a widely used organic solvent in chemical industries. It is used as a solvent for acetylene and semi-synthetic polymers like nitrocellulose, cellulose acetate etc. It is also used to dissolve varnishes and polishing materials.
14. Write some uses of kerosene as a solvent.
Ans. Kerosene is an important organic solvent. It is used to dissolve different organic substances as well as colours. It can also remove colour stains from clothes.
15. Write some uses of chloroform as a solvent.
Ans. Chloroform is an excellent organic solvent. In different industries, it is used to dissolve substances like fat, rubber, wax, resin etc. It is also widely used in the extraction of oils, gums and alkaloids.
16. Why are volatile solvents used to dissolve colours and varnishes?
Ans. Colours and varnishes are generally dissolved in volatile solvents because of the following reasons-
- When the colour or varnish is applied on a surface, the volatile solvent evaporates easily. As a result, a smooth layer of colour or varnish is obtained.
- When a colour is sprayed by dissolving it in a volatile solvent, less amount of colour is required thus, making the process more costeffective.
17. Discuss some harmful effects of using volatile solvents.
Ans. In our everyday life, we come across a number of volatile solvents. Volatile organic solvents like methyl alcohol, methylated spirit, turpentine or tarpin oil, naphtha, acetone etc. are widely used as solvents for oil paints, paint thinners, spray paints, varnishes etc. Being volatile in nature, these solvents evaporate very fast even at ordinary temperature and can enter our body through respiratory system and get absorbed in the blood through our lungs. In fact, these solvents may directly come in contact with our skin. Thus exposure to these solvent may cause serious damage to our body. The harmful effects caused by volatile solvents can be categorised into two groups- (1) harmful effects due to short-term exposure and (2) harmful effects due to long-term exposure.
Harmful effects due to short term exposure to volatile solvents include- (1) allergies, (2) headache, (3) asthma, (4) nausea and vomiting tendency, (5) drowsiness, (6) burning sensation in respiratory tract eyes, nose etc.
Harmful effects due to long term exposure to volatile solvents include- (1) increased risk of cancer, (2) damage of kidneys and liver, (3) delayed childhood development and (4) damage of central nervous system.
18. Mention the harmful effects of ethyl alcohol, methyl alcohol and acetone when they are used as solvents.
Ans. Harmful effects of ethyl alcohol: It is used as a solvent for different medicines. On entering the human body, it affects the nervous system. Thus, transmission of nervous impulse across the body gets disrupted and the person suffers from dizziness. Ethyl alcohol also reacts with some medicines and cause adverse side-effects.
Harmful effects of methyl alcohol: Methyl alcohol (CH3OH) is a volatile toxic compound and it decomposes to form formaldehyde (HCHO). If 10 mL CH3OH enters into the body, it may cause blindness due to the effect of HCHO, if 30 mL enters, the person may become unconscious and if 100 mL enters the body, the person may even die. Harmful effects of acetone: If excess acetone enters into the body through respiration, then it may lead to temporary nervous breakdown.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. Brownian motion of colloidal particles is caused due to
A. convection
B. attraction between the particles of dispersed phase and dispersion medium
C. unequal collision between the particles of dispersed phase and dispersion medium
D. change in temperature
Ans. C
2. The correct order for diffusion is
A. solid > liquid > gas
B. liquid > solid > gas
C. gas > solid > liquid
D. gas > liquid > solid
Ans. D
3. The Brownian motion of colloidal particles indicates
A. linear motion
B. circular motion
C. spiral motion
D. random motion
Ans. D
4. Which of the following is not an organic solvent?
A. liquid ammonia
B benzene
C. chloroform
D. acetone
Ans. A
5. Which of the following substances cannot form crystals by sublimation?
A. iodine
B. ammonium chloride
C. sodium chloride
D. benzoic acid
Ans. C
6. Just before crystallisation, the solution somewhat becomes
A. saturated
B. unsaturated
C. supersaturated
D. precipitated
Ans. C
7. An example of a crystalline solid is
A. glass
B. wax
C. pitch
D. common salt
Ans. D
8. An example of an amorphous solid is
A. sugar
B. salt
C. glass
D. blue vitriol
Ans. C
9. Which of the following is a method of crystallisation?
A. sublimation
B. filtration
C. fractional distillation
D. decantation
Ans. A
10. Mixing of the solute and solvent particles due to continuous, random motion of the particles is known as
A. osmosis
B. diffusion
C. effusion
D. distillation
Ans. B
11. An example of an organic solvent is
A. water
B. liquid ammonia
C. acetone
D. liquid SO2
Ans. C
12. An inorganic solvent except water is
A. chloroform
B. kerosene
C. liquid ammonia
D. ethyl alcohol
Ans. C
13. An example of lyophilic sol is
A. gold sol
B. silver sol
C. sulphur sol
D. starch sol
Ans. D
14. An example of a lyophobic sol is
A. ferric hydroxide sol
B. starch sol
C. gelatin
D. soap
Ans. A
15. In which of the following diffusion does not take place?
A. hydrogen and nitrogen
B. oxygen and water
C. NaCl and Na2SO4
D. Sugar and water
Ans. C
16. In which of the following, there is no water of crystallisation?
A. blue vitriol
B. borax
C. washing soda
D. sugar
Ans. D
17. Anhydrous copper sulphate, by nature, is
A. deliquescent
B. hygroscopic
C. supersaturated
D. none of these
Ans. B
18. The residual solution after separating the crystal by crystallisation is
A. saturated
B. unsaturated
C. supersaturated
D. colloid
Ans. A
19. Inorganic, non-aqueous solvent is
A. chloroform
B. liquid CO2
C. ethanol
D. kerosene
Ans. B
20. Universal solvent is
A. alcohol
B. water
C. ether
D. all of the above
Ans. B
21. Which solvent is used to remove nail polish?
A. ethyl alcohol
B. methyl alcohol
C. acetone
D. tarpene oil
Ans. C
22. Crystal without water of crystallisation is
A. common salt
B. blue vitriol
C. alum
D. epsom salt
Ans. A
Answer in brief
1. Define water of crystallisation.
Ans. One or more than one water molecule attached to the crystals of certain compounds on which the colour and shape of the crystal largely depend is known as water of crystallisation.
2. Write the formula of common alum.
Ans. The formula of common alum is K2SO4 · Al2(SO4)3 · 24H2O.
3. Why does diffusion take place in a solution?
Ans. Diffusion takes place due to continuous random motion of solute and solvent particles in a solution.
4. What is the effect of temperature on diffusion of a liquid?
Ans. With the rise in temperature, diffusion in a liquid occurs at a faster rate.
5. Give some examples of organic solvents.
Ans. Ethyl alcohol, methyl alcohol, acetone, chloroform, kerosene, carbon tetrachloride etc., are some examples of organic solvents.
6. Give some examples of inorganic solvents other than water.
Ans. Some examples of inorganic solvents other than water are liquid ammonia, liquid nitrogen dioxide, liquid sulphur dioxide etc.
7. Name a poisonous organic solvent.
Ans. Methyl alcohol
8. Name an organic solvent which is actually a mixture.
Ans. Kerosene is an organic solvent which is actually a mixture of hydrocarbons.
9. Which volatile solvent acts as a anaesthetic?
Ans. Chloroform is used as anaesthetic.
10. Give an example of a solvent which can dissolve both of sulphur and phosphorous.
Ans. Carbon disulphide (CS2).
11. Name two non-aqueous solvent.
Ans. Ethyl alcohol and chloroform.
12. Name a crystal containing water of crystallisation.
Ans. Hypo sodium or (Na2S2O3 · 5H2O). thiosulphate
13. Name the solvent used in paint and varnish.
Ans. Methylated spirit, tarpene oil, naptha, acetone etc.
14. Give an example of efflorescent crystal.
Ans. Blue vitriol or blue stone i.e. CuSO4, 5H2O is an example of efflorescent crystal.
15. What happens when blue vitriol is heated over 250°C?
Ans. Water of crystallisation of blue vitriol crystal evaporated upon heating over 250°C to produce white powder of amorphous copper sulphate.
16. Mention the number of water of crystallisation in common alum.
Ans. 24
Fill in the blanks
1. The size of a crystal depends upon the ……….. and ………… of the constituent atoms or particles present in the crystal. com
Ans. size, arrangement
2. Glass is a/an ……….. solid.
Ans. amorphous
3. Acetone is an example of ……….. solvent.
Ans. organic
4. Number of water of crystallisation in glauber salt is ………..
Ans. 10
5. Example of a crystalline compound without water of crystallisation is ………..
Ans. sodium chloride
State whether true or false
1. Brownian motion of colloidal particles is continuous and random in nature.
Ans. True
2. In diffusion, the solute particles move from a region of higher concentration to lower concentration.
Ans. True
3. Methyl alcohol on entering the body decomposes to form acetaldehyde.
Ans. False
4. Chloroform is a volatile solvent which is oxidised to form the toxic compound, phosgene.
Ans. True
5. Seed crystals can accelerate the process of crystallisation of gemstones.
Ans. True
6. Sublimation is a method of crystallisation.
Ans. True