WBBSE 9th Class Science Solutions Physical Science & Environment Chapter – 4.5 Separation of the Components of Mixture
West Bengal Board 9th Class Science Solutions Physical Science & Environment Chapter – 4.5 Separation of the Components of Mixture
WBBSE 9th Class Physical Science & Environment Solutions
Synopsis
- A homogeneous mixture is one in which the mixture has the same proportion of the components throughout and has uniform properties at all parts of the mixture.
- To separate useful substances from a mixture or to eliminate unwanted or harmful substances from a mixture, the components of a mixture need to be separated. Different methods applied for separation of mixtures are-crystallisation, distillation, fractional distillation, sublimation, filtration, chromatography etc.
- Crude petroleum is a liquid mixture of hydrocarbons containing 1-40 carbon atoms (C₁-C40). Considering its immense importance in our everyday life, petroleum is often referred to as ‘liquid gold’. Crude petroleum is refined by the process of fractional distillation.
- The four main parts obtained from fractional distillation of petroleum are- (1) crude naphtha, (2) kerosene or paraffin oil, (3) fuel oil or diesel, (4) residual oil.
- The boiling point of a liquid increases when pressure above the surface of the liquid increases, i.e., the liquid boils at a higher temperature compared to its normal boiling point.
- The process of distillation is applied to . separate the components of a mixture consisting of a non-volatile solid dissolved in a liquid. It is used mainly to separate the liquid component. For example, iodine and chloroform can be separated from their mixture by distillation. This process is extensively used in the purification of substances by solvent extraction method. The components of a homogeneous mixture consisting of two liquids can be separated by simple distillation if- (1) the difference in boiling points of the two liquids is greater than 30°C and (2) the liquids do not decompose or react with each other on heating. Thus, separation of benzene (boiling point = 80°C) and aniline (boiling point = 184°C) from their mixture may be done by simple distillation.
- When a mixture of two liquids with different boiling points is heated at a constant pressure, the relative proportion of the more volatile component is greater in the formed vapour than the relative proportion of that component present in the liquid mixture. If the difference in boiling points of the liquids is more than 30°C, the vapour phase mostly contains the more volatile component (i.e. liquid with the lower boiling point) when the liquid mixture is heated around the boiling point of the more volatile component.
TOPIC – A
Necessity of Separation of the Components of a Mixture and Purification of Petroleum
SHORT AND LONG ANSWER TYPE QUESTIONS
1. Define mixture. Classify mixture into different types.
Ans. A mixture is the physical combination of two or more substances combined in any possible proportion by mass, in which the identities of the components are retained.
Mixtures may be classified in two types- (1) homogeneous mixture and (2) heterogeneous mixtures.
2. What is a homogeneous mixture? Give example.
Ans. A homogeneous mixture is one in which the components of the mixture has the same proportion throughout and has uniform properties at all parts of the mixture.
Aqueous solution of sugar, aqueous solution of alcohol etc. are homogeneous mixtures.
3. Define heterogeneous mixtures.
Ans. A heterogeneous mixture is a mixture with a non uniform composition. The composition varies from one region to another with at least two phases that remain separate from each other, with clearly identifiable properties. e.g. mixture of sand and water, mixture of chalk-dust and water. Concrete is a heterogeneous mixture of cement and water.
4. Write the differences between homogeneous mixtures and compounds.
Ans. The major differences between homogeneous mixtures and compounds are as follows-
| Homogeneous mixture |
Compound |
| 1. In a homogeneous mixture, the components may be present in any ratio. |
1. In a compound, the constituent elements are always present in a definite ratio by mass. |
| 2. The components of a homogeneous mixture retain their individual properties. |
2. The constituent elements of a compound lose their individual properties. |
| 3. The components of a homogeneous mixture can be separated by simple physical processes. |
3. The constituents of a compound cannot be separated by simple physical processes. |
5. Why is it necessary to separate the components of a mixture?
Ans. (1) Separation methods are necessary for the removal of unwanted and harmful components from a mixture. For example, removal of impurities like soil particles, dirt. etc. from water makes it suitable for drinking.
(2) Useful components can be obtained from a mixture by applying different separation techniques. For example, petrol, diesel, kerosene, etc. are obtained from crude petroleum by fractional distillation.
6. What is petroleum? In which process its components can be separated?
Ans. Petroleum is a naturally occurring sticky liquid with characteristic smell, found. beneath the earth’s surface and is a mixture of hydrocarbons. containing 1 to 40 number of carbon atoms.
In petroleum, a mixture of hydrocarbons with boiling points in the range of 20°C-400°C are present. That is why to separate different components of it, fractional distillation is used.
7. Why is it necessary to separate the components of petroleum?
Ans. Petroleum is a mixture of different hydrocarbons containing 1 to 40 carbon atoms. It is the major source of different fuels like petrol, diesel, kerosene and a number of useful chemicals like lubricating oil, bitumen, paraffin wax etc. These substances are widely used in different household activities as well as in industries. Thus, refining of crude petroleum i.e., separation of the components of petroleum is necessary.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. During fractional distillation of crude petroleum into its constituents, petrol distils at
A. 70°C
B. 120°C
C. 250°C
D. 400°C
Ans. A
2. The correct order of density of (i) ether, (ii) petrol and (iii) kerosene is
A. (i) < (iii) < (ii)
B. (i) < (ii) > (iii)
C. (iii) > (ii) > (i)
D. (i) > (ii) > (iii)
Ans. C
3. Which of the following is a homogeneous mixture?
A. water and oil
B. water and alcohol
C. water and sand
D. water and benzene
Ans. B
4. The component of crude petroleum which gets distilled at the lowest temperature is
A. bitumen
B. refinery gas
C. naphtha
D. kerosene
Ans. B
5. The component of crude petroleum which gets distilled at the highest temperature is
A. bitumen
B. diesel
C. gasoline
D. kerosene
Ans. A
6. Which of the following is not obtained due to fractional distillation of petroleum?
A. kerosene
B. naphtha
C. bitumen
D. coal gas
Ans. D
7. The component of petroleum which is not used as a fuel is
A. petrol
B. naphtha
C. kerosene
D. diesel
Ans. B
8. The temperature at which LPG is obtained during fractional distillation of petroleum is
A. below 30°C
B. above 30°C
C. above 50°C
D. 95°-100 °C
Ans. A
9. Which of the following is obtained from the fractional distillation of petroleum
A. polythene
B. ammonia
C. diesel
D. teflon
Ans. C
10. Diesel is obtained by fractional distillation of petroleum at
A. 70°C
B. 170°C
C. 270°C
D. 370°C
Ans. C
11. Which one of the following is not a homogeneous mixture?
A. mixture of water and common salt
B. mixture of sugar and water.
C. mixture of water and alcohol
D. blood
Ans. D
12. Which one of the following is not a heterogeneous mixture?
A. blood
B. milk
C. muddy water
D. air
Ans. D
Answer in brief
1. Between compounds and mixtures, the components of which one can be separated by simple physical processes?
Ans. The components of a mixture can be separated by simple physical processes.
2. Give an example of a homogeneous mixture.
Ans. An aqueous solution of sugar.
3. A mixture consists of finely ground wheat flour and sugar. Different parts of the mixture are tasted but the sweetness of the mixture is not uniform throughout the mixture. What may be the reason?
Ans. The sweetness of the mixture is not uniform throughout the mixture because it is a heterogeneous mixture.
4. Which process is used in the refining of crude petroleum?
Ans. Fractional distillation.
5. Is it possible to separate the constituents of petroleum by distillation?
Ans. No, it is not possible to separate the constituents of petroleum by distillation.
6. What is the basis of separation of the components of crude petroleum?
Or, By which physical property the components of petroleum can be differentiated?
Ans. The different components of crude petroleum are separated on the basis of their difference in boiling points by fractional distillation method.
7. Name a solid substance obtained from the fractional distillation of petroleum.
Ans. Paraffin.
8. What is petroleum gas?
Ans. The hydrocarbon filtrate part containing 1 to 4 carbon atoms, obtained by fractional distillation of petroleum at 20°C to 30°C is termed as petroleum gas.
9. What is the main component of petroleum gas?
Ans. Butane.
10. What is asphalt?
Ans. The black, sticky residue obtained from fractional distillation of petroleum is termed as asphalt.
11. How is gasoline generally known to us?
Ans. Gasoline is known as petrol.
12. Arrange in ascending order of boiling point-petrol, diesel, lubricating oil and kerosene.
Ans. Petrol < kerosene < diesel < lubricating oil.
13. Name some liquid fuels obtained from petroleum by fractional distillation.
Ans. Petrol or gasoline, diesel and kerosene.
14. What is obtained at lowest temperature in the refinement of petroleum?
Ans. Petroleum gas is obtained at lowest (20°C) temperature.
Fill in the blanks
1. The component of petroleum which is used in construction of roads is ………….
Ans. asphalt
2. The other name of petrol is ………….
Ans. gasoline
3. The main component of petroleum gas is ………….
Ans. butane
4. …………. gas is used as household fuel for cooking.
Ans. Petroleum
5. Petroleum is purified by ………….
Ans. fractional distillation
State whether true or false
1. Naphtha obtained from fractional distillation of petroleum is used as a fuel.
Ans. False
2. Bitumen is obtained as residue in the fractional distillation of petroleum.
Ans. True
3. Milk of magnesia is an example of homogeneous mixture.
Ans. False
4. Coal tar is obtained by the destructive distillation of bituminous coal.
Ans. True
5. Crude petroleum oil can be used as fuel.
Ans. False
6. Petroleum consists of more than 150 types of hydrocarbons.
Ans. True
7. Kerosene is used as a solvent of DDT.
Ans. True
TOPIC – B
Distillation, Fractional Distillation, Separatory Funnel
SHORT AND LONG ANSWER TYPE QUESTIONS
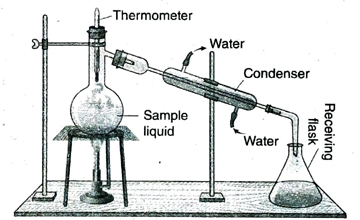
1. What is distillation?
Ans. The process by which a mixture consisting of a liquid and dissolved solid substances is vaporised by applying heat, followed by condensing the vapour back into the liquid state, thereby separating the components of the mixture is called distillation.
2. What is the working principle of distillation?
Ans. The process of distillation is quite useful for separating the components of a mixture consisting of two miscible liquids, the difference in their boiling points being greater than 30°C. This process is also effective in separating the components from a mixture consisting of a liquid and non-volatile solid impurities.
When the solution is heated around the boiling point of the more volatile component, the vapour phase formed contains the more volatile component in a relatively higher proportion. The vapour is then condensed to the corresponding liquid and collected in a beaker. When the temperature is raised to the boiling point of the less volatile component, the vapour phase formed contains the less volatile component in relatively higher proportion because the more volatile component has almost completely distilled off at a lower temperature. Thus in distillation, the liquids distill off from the mixture at their respective boiling points as vapours and the vapours are then condensed to liquid phase and collected in beakers. The most volatile component distills off first while the least volatile component distills off at the end.
3. which type of mixtures are separated by distillation?
Ans. Distillation is used to separate a homogeneous mixture of a solid dissolved in a liquid and to separate the components of a mixture consisting of two miscible liquids if the difference in boiling points of the liquids is in the range of 30-50°C and the liquids do not decompose on heating.
4. What is Liebig condenser?
Ans. The Liebig condenser or straight condenser is a piece of laboratory equipment, consisting of a straight glass tube surrounded by a water jacket in which water is constantly circulated to carry away the heat of vaporisation released by the condensing vapour.
5. Give an example of a natural distillation process.
Ans. Sea water evaporates leaving behind the salts in the sea. Water vapours obtained as a result of such evaporation go upward and form clouds. From these clouds when water comes down to earth as rain, it does not contain any salt in it. Therefore the water cycle may be cited as an example of natural distillation process.
6. What is vacuum distillation? When this process is applied??
Ans. Vacuum distillation is distillation performed under reduced pressure, which allows the purification of compounds not readily distilled at ambient pressures or simply to save time or energy. This process allows the components to be distilled at a lower temperature than their boiling points.
Vacuum distillation is ideal for separating mixture of liquids with very high boiling points. The lowering of pressure enables the components to boil at lower temperature. Once the vapour pressure of the component is equal to the surrounding pressure, it is converted to vapour, which are then condensed and collected as distillate. The vacuum distillation is also used to obtain high-purity samples of compounds that decompose at high temperatures.
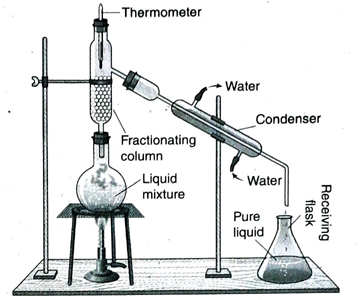
7. What is fractional distillation?
Ans. The process of separation of a mixture consisting of two miscible liquids having a difference of boiling point less than 25°C, by repeated condensation and vapourisation in a fractionating column is known as fractional distillation.
8. Write down the basic principle of he basic principle of fractional distillation.
Ans. Fractional distillation is used to separate a mixture of two or more miscible liquids which boil without decomposition and for which the difference in boiling points is less than 25°C. If a mixture of such kind is heated, the liquid with the least boiling point evaporates first and its vapour is transformed to pure liquid by condensation. By increasing the temperature slowly, other components of the mixture are obtained in their pure form.
9. Under which conditions, fractional distillation is used instead of simple distillation?
Ans. Simple distillation method is not suitable for separation of components of a mixture containing two or more miscible liquids having a difference in boiling point less than 25°C. The more volatile component vapourises when the mixture is heated near its boiling point. However, as the difference in boiling point is small, other less volatile components also vapourise in small amounts and deposit in the receiving flask. Thus, complete separation of the components of the mixture does not take place by simple distillation. So, fractional distillation is done in which the mixture by repeated vaporisation and condensation in the fractionating column separates into its components.
10. State important applications of fractional distillation.
Ans. (1) Crude petroleum is refined by fractional distillation to get different components like petrol, diesel, kerosene, lubricating oil etc.
(2) Different gaseous components (oxygen, nitrogen etc.) are separated from liquid air by the process of fractional distillation.
(3) Benzene is separated from coal tar, using fractional distillation.
(4) From a mixture of acetone and methyl alcohol components are separated using fractional distillation.
11. How will you separate benzene (bp 80°C) and toluene (bp 110°C) form a mixture?
Ans. Benzene and toluene can be separated from their mixture by fractional distillation.The mixture should be taken in a distillation flask and thermometer, fractionating column, condenser etc. should be attached as per standard procedure. The flask should be hosted in an oil bath. AT 80°C boiling starts and the temperature remains constant. At this temperature benzene vapour passes through the fractionating column and the condenser, condensed to liquid form and collected. After complete distillation of benzene, temperature of the mixture starts to increase. At 110°C toluene starts to vapourise and after passing through the fractionating column and the condenser, collected as liquid toluene.
12. What are the limitations of fractional the limitations of fractional distillation?
Ans. The process of fractional distillation cannot be used to separate the components of an azeotropic mixture (constant boiling mixture). For example, a mixture of 95.6% ethyl alcohol and 4.4% water cannot be separated by fractional distillation because it forms a constant boiling mixture or azeotropic mixture which boils at a constant temperature of 78.4°C.
13. What is azeotropic mixture?
Ans. Azeotropic mixture is the mixture of miscible liquids that has a constant boiling point because the vapour has the same composition as the liquid mixture. The boiling point of an azeotropic mixture may be higher or lower than that of any of its components.
14. Why a mixture of water and ethyl alcohol can not be separated completely by fractional distillation?
Ans. While concentrating a mixture of ethanol and water through fractional distillation, a mixture of 95.6% ethanol and 4.40% water (by weight) is obtained at some point. On heating, at 78.4°C the whole liquid starts boiling and distilled in the same composition. In fact, at that composition ethanol and water forms an azeotrope. That is why complete separation of ethanol or ethyl alcohol and water from their mixture can not be done through fractional distillation.
15. How is the boiling point of a liquid related to the pressure above its surface? Write important applications of this property.
Ans. (1) The boiling point of a liquid increases if the pressure above its surface increases. Thus, the liquid boils at a higher temperature than its normal boiling point.
Application: This property of liquid is applied in pressure cookers.
(2) The boiling point of a liquid decreases if the pressure above its surface decreases. Thus, the liquid boils at a lower temperature than its normal boiling point.
Application: Condensed milk is prepared by using this property. Milk is condensed by boiling it at a low temperature by decreasing the pressure.
16. Write down the effect of pressure on of pressure of boiling point of liquids.
Ans. Boiling point of liquids depend on the pressure exerted on the liquid. As the pressure increases, boiling point of liquids also increases and decreases with decrease in pressure.
17. Give two examples where separatory funnel is used to separate the components of a mixture.
Ans. (1) Separatory funnel is used to separate the components of a mixture of two immiscible liquids like oil and water, benzene and water, chloroform and water etc.
(2) Separatory funnel is used to extract an organic compound dissolved in water by solvent extraction process.
18. How water and carbon tetrachloride are separated from their mixture?
Ans. The mixture of water and carbon tetrachloride is poured and kept for sometime in a separatory funnel. Carbon tetrachloride forms the lower layer and water forms the upper layer in the separatory funnel. Carbon tetrachloride is collected first by opening the stopcock and then water is collected in a separate vessel.
19. Is it possible to separate water and alcohol from their mixture with the help of a separatory funnel?
Ans. Liquids which are completely miscible with each other and forms a homogeneous mixture cannot be separated from their mixture with the help of a separatory funnel. Water and alcohol completely mix with each other in all proportions to form a homogeneous mixture. So, separatory funnel cannot be used to separate water and alcohol from their mixture.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. Which of the following is not a compound?
A. benzene
B. water
C. petroleum
D. toluene
Ans. C
2. The process of distillation can be used to separate the
A. constituents of a mixture of water and salt
B. constituents of a mixture of water and oil
C. constituents of a mixture of water and sand
D. constituents of a mixture of sugar and salt
Ans. A
3. Which of the following mixtures cannot be separated into its constituents by using a separatory funnel?
A. water and mercury
B. petrol and water
C. chloroform and water
D. ethanol and water
Ans. D
4. A fractionating column is used for the process of
A. filtration
B. distillation
C. vapourisation
D. fractional distillation
Ans. D
5. Benzene and toluene can be separated from their mixture by
A. filtration
B. distillation
C. fractional distillation
D. using separatory funnel
Ans. C
6. Which of the following process of separation of a mixture does not require heating?
A. distillation
B. sublimation
C. using separatory funnel
D. fractional distillation
Ans. C
7. Water can be boiled at room temperature by
A. decreasing the pressure
B. increasing the pressure
C. initially increasing the pressure and then decreasing it
D. initially decreasing the pressure and then increasing it
Ans. A
8. During fractional distillation of two miscible liquids, the distilled vapour produced on heating the mixture is rich in
A. less volatile liquid
B. more volatile liquid
C. both the liquids
D. none of the liquids
Ans. B
9. Which of the following is required in solvent extraction method?
A. filter paper
B. funnel
C. separatory funnel
D. beaker
Ans. C
10. Benzene and nitrobenzene can be separated from their mixture by
A. fractional distillation
B. filtration
C. simple distillation
D. sublimation
Ans. C
11. The glass apparatus which is used to separate the components of a mixture consisting of two or more immiscible liquids is called
A. fractionating column
B. condenser
C. separatory funnel
D. Buchner funnel
Ans. C
12. A salt can be extracted from its aqueous solution by
A. sublimation
B. filtration
C. distillation
D. fractional distillation
Ans. C
13. For every 27 mm increase in atmospheric pressure, the boiling point of water increases by
A. 1°C
B. 2°C
C. 3°C
D. 4°C
Ans. A
14. The boiling point of water at Darjeeling will be
A. more than 100°C
B. less than 100°C
C. equal to 100°C
D. cannot be determined
Ans. B
15. The process of separation of components from the mixture of chloroform (boiling point 61°C) and benzene (boiling point 80°C) is
A. distillation
B. fractional distillation
C. separating funnel
D. filtration
Ans. B
16. From which of the following mixtures, components cannot be separated by fractional distillation?
A. water + ethyl alcohol (bp: 78.2°C)
B. water + kerosene (bp: 170°C)
C. acetone (bp:56°C)+ methanol (bp: 65°C)
D. water + benzene (bp: 80°C)
Ans. A
17. The process by which the components of a mixture of ethanol and water can be separated is
A. distillation
B. fractional distillation
C. sublimation
D. none of the above
Ans. D
18. The process by which the components of a mixture of ammonium chloride and sand can be separated is
A. distillation
B. fractional distillation
C. sublimation
D. chromatography
Ans. C
19. The process used to separate the gaseous components of air is
A. distillation
B. fractional distillation
C. sublimation
D. separating funnel
Ans. B
20. Water can be boiled at 25°C if the standard atmospheric pressure is
A. reduced
B. increased
C. increased at first and then reduced
D. reduced at first and then increased
Ans. A
21. The process of separation of a liquid and a non-volatile solid from their mixture is
A. fractional distillation
B. sublimation
C. distillation
D. crystallisation
Ans. C
22. The process used to prepare salt from sea water is
A. distillation
B. evaporation
C. fraction distillation
D. filtration
Ans. B
23. Apparatus used in fractional distillation is
A. condenser
B. Liebig’s condenser
C. fractional column
D. B and C both
Ans. D
24. Which one of the following cannot be obtained by the fractional distillation of crude petroleum?
A. petrol
B. diesel
C. ethanol
D. kerosene
Ans. C
25. How will you separate the components of a mixture of iodine and ethanol ?
A. filtration
B. evaporation
C. distillation
D. fractional distillation
Ans. C
26. Distillation column is used in
A. distillation
B. fractional distillation
C. condensation
D. separatory funnel
Ans. C
27. By which process water and carbon tetrachloride can be separated from their mixture?
A. distillation
B. fractional distillation
C. evaporation
D. separatory funnel
Ans. D
28. The components of a mixture of water and kerosene, can be separated by
A. distillation
B. fractional distillation
C. separatory funnel
D. sublimation
Ans. C
Answer in brief
1. What are the processes of separation of component of a mixture of two liquids?
Ans. Distillation, fractional distillation, separating funnel etc.
2. What change is observed in the boiling point of a liquid when the pressure above its surface is decreased?
Ans. When the pressure above the surface of a liquid is decreased, the liquid starts. to boil at a lower temperature, i.e., the boiling point of the liquid decreases.
3. What should be the minimum difference in the boiling points of two liquids if they are to be effectively separated by the process of distillation?
Ans. If the difference in the boiling points of two liquids is about 30-50°C, then they can be effectively separated by the process of distillation.
4. Which constituent liquid will be in relatively higher amount in the vapour phase when a mixture containing two miscible liquids is heated during fractional distillation?
Ans. In fractional distillation of a mixture of two miscible liquids, the vapour phase will contain the more volatile liquid in relatively higher amount when the mixture is heated.
5. How can a mixture of sand and iron dust be separated?
Ans. A mixture of sand and iron dust can be effectively separated by using a magnet. The process is called magnetic separation.
6. Define sublimation.
Ans. Sublimation is the transition of a substance directly from the solid to the gaseous state, by the application of heat, without passing through the liquid state.
7. In which process pure water can be obtained from an aqueous solution of salt?
Ans. By the process of distillation.
8. By which process the components of a mixture of methanol (bp: 65°C) and water can be separated?
Ans. By the process of distillation.
9. In which process the components of the mixture of ether (bp: 35°C) and benzene (bp: 80°C) can be separated?
Ans. Distillation.
10. When is fractional distillation used for separating the components of a mixture?
Ans. If the difference in boiling points of two miscible liquids is less than 25°C, then those liquids are separated from their mixture by fractional distillation.
11. Which apparatus is effectively used in fractional distillation of a mixture containing two or more miscible liquids, the difference in their boiling points being less than 25°C ?
Ans. The apparatus which is effectively used in fractional distillation of a mixture containing two or more miscible liquids (the difference in their boiling points being less than 25°C), is a fractional distillation column or fractionating column.
12. Which process is suitable to separate acetone and methanol from their mixture?
Ans. Acetone (boiling point = 56°C) and methanol (boiling point = 65°C) can be separated from their mixture by the process of fractional distillation.
13. Is it possible to separate the components of a mixture containing two or more miscible liquids by distillation if the difference in their boiling points is 25°C?
Ans. No, it is not possible.
14. Mention the percent amount of ethyl alcohol in rectified spirit which is an azeotropic mixture.
Ans. Rectified spirit which in an azeotropic mixture contains 95.6% ethyl alcohol.
15. What is the boiling point of rectified spirit which is an azeotropic mixture?
Ans. The boiling point of rectified spirit which is an azeotropic mixture is 78.4°C.
16. Two immiscible liquids, water and kerosene are kept in a beaker for some time. Which liquid will form the lower layer?
Ans. As water is heavier than kerosene, it will form the lower layer.
17. Which process is suitable to separate water and kerosene from their mixture?
Ans. Water and kerosene can be separated from their mixture by using a separatory funnel.
18. Is it possible to separate water and sand from their mixture by using a separatory funnel?
Ans. Water and sand cannot be separated from their mixture by using a separatory funnel.
19. State whether fractional distillation is a physical or chemical process.
Ans. Fractional distillation is a physical process of separation of two or more miscible liquids.
20. Which one of methanol and acetone will distill first if their mixture is being heated in a distillation flask?
Ans. Boiling points of methanol and acetone are 78.5°C and 56°C respectively. So acetone will distill first if their mixture is being heated in a distillation flask.
21. Why the components of a mixture of methanol and acetone can not be separated by simple distillation?
Ans. The difference in boiling point of methanol (bp: 65°C) and acetone (bp: 56°C) is too small (9°C) to separate them by simple distillation. To separate the components from a mixture by simple distillation, difference in boiling point should be at least 30°C.
22. Give example of azeotropic mixture.
Ans. The mixture of 95.6% ethanol and 4.4% water, is an example of azeotropic mixture.
23. Name the suitable separation technique by which a mixture of two liquids of different boiling points can be separated.
Ans. Distillation or fractional distillation depending upon the difference in boiling points of the component liquids.
24. By which process water and benzene can be separated from their mixture?
Ans. Using separatory funnel.
25. Write down two essential conditions for separation of liquid mixture through separatory funnel?
Ans. (1) Density of the liquids should be different.
(2) The liquids should be immisscible.
26. How will you separate the components of a mixture containing water and kerosene or water and petrol?
Ans. By using separatory funnel.
27. Name the separation technique by which the mixture of two immissible liquids can be separated.
Ans. By using separatory funnel.
28. Name a liquid mixture the components of which can not be separated by separatory funnel.
Ans. A mixture of water and ethyl alcohol.
Fill in the blanks
1. A liquid starts to boil when the vapour pressure of the liquid is equal to the ……….. pressure.
Ans. atmospheric
2. Two miscible liquids can be separated from their mixture by distillation they do not ………. on heating.
Ans. decompose
3. During separation of a mixture consisting of two or more miscible liquids by fractional distillation, the liquid with the ………… boiling point will distill out first.
Ans. lowest
4. The different gaseous constituents of air is separated by ………….
Ans. fractional distillation
5. Water can be boiled at room temperature (25°C) if the pressure on water is …………. than the atmospheric pressure.
Ans. less
6. Ether and toluene can be separated from their mixture by …………
Ans. distillation
7. The process which is widely used in purification of substances by solvent extraction method is ………..
Ans. distillation
8. Sugar is separated from its aqueous solution by ………….. process.
Ans. distillation
9. Liquids that do not vapourise easily are called ………….. liquids.
Ans. non-volatile
10. In an azeotropic mixture of rectified spirit, the percentage of water is ………….
Ans. 4.4%
11. In a fractionating column, as the vapour moves up in the column, the relative amount of the …………. volatile component in the vapour increases.
Ans. more
12. In a fractionating column, the process of condensation and vapourisation take place ………….. throughout the column.
Ans. repeatedly
13. Water and alcohol ………….. be separated completely from their mixture by fractional distillation method.
Ans. cannot
14. The gaseous components of …………. air are separated by fractional distillation.
Ans. liquid
15. A mixture of water and mustard oil can be separated by using a …………..
Ans. separatory funnel
16. Benzene is extracted from coal tar by the process of …………..
Ans. fractional distillation
17. Two liquids cannot be seperated by fractional distillation if their mixture forms an …………..
Ans. azeotrope
18. To cool down the vapours of the distilled liquid in distillation, ……….. condenser is used.
Ans. Liebig
19. Distillation = Evaporation + ……………..
Ans. Condensation
20. In a mixture of water and chloroform, ………….. forms the lower layer.
Ans. chloroform
State whether true or false
1. If the difference in the boiling points of two liquids is 30°C, then those liquids can be separated from their mixture by the process of simple distillation.
Ans. True
2. Fractional distillation is a chemical process of separation of mixtures.
Ans. False
3. A mixture of water and benzene forms a homogeneous mixture.
Ans. False
4. Two immiscible liquids of different densities can be separated from their mixture by a separatory funnel.
Ans. True
5. The different components of liquid air are separated by the process of fractional distillation.
Ans. True
6. Boiling point of a liquid decreases if pressure above the liquid is increased.
Ans. False
7. A mixture of sand and iron dust is separated by magnetic separation.
Ans. True
8. It is not possible to boil water at room temperature.
Ans. False
9. Azeotropic mixtures cannot be separated by fractional distillation.
Ans. True
10. During fractional distillation of two miscible liquids, the less volatile component remains in the vapour phase in greater proportion.
Ans. True
11. Water forms the upper layer when it is kept with kerosene in a beaker.
Ans. False
12. During the process of distillation, the most volatile component distils out last while the least volatile component distils out first.
Ans. False
13. A mixture of water and alcohol can be separated by using a separatory funnel.
Ans. False
14. Fractional distillation is used to separate salt from sea water.
Ans. False
15. Boiling point of methanol is greater than that of water.
Ans. False
16. Boiling point of liquid decreases by decreasing pressure.
Ans. True
17. In a mixture of carbon disulphide and water, carbon disulphide constitutes the lower layer.
Ans. True
18. The mixture of acetone and water can be separated using separatory funnel.
Ans. False
19. Colloid solutions can be separated by separatory funnel.
Ans. False