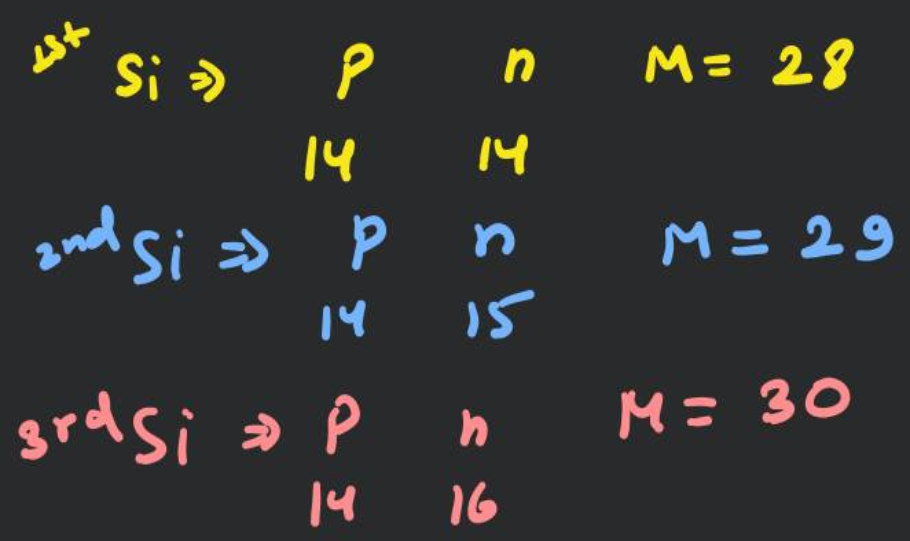
Atomic number of silicon is 14. If there are three isotopes of silicon having 14,15 and 16 neutrons in their nuclei, what would be the symbol of the isotope?
Atomic number of silicon is 14. If there are three isotopes of silicon having 14,15 and 16 neutrons in their nuclei, what would be the symbol of the isotope?