Carbon and its Compounds
Carbon and its Compounds
Carbon is non-metal having atomic number 6 and mass number 12. It is placed in group (IV) A or group 14 in periodic table. Valency of carbon is 4.
Allotropy
The substances which have same chemical properties, but different physical properties are called allotropes and this property is called allotropy. Example-Allotropies of Carbon-Diamond, graphite, charcoal.
Diamond
1. It is the purest form of carbon.
2. It is the hardest natural known substance.
3. It is transparent, and specific gravity 3.52.
4. It is bad conductor of electricity and heat.
5. It has very high refractive index 2.415.
6. It is chemically inert and on heating above 1500° C, transferred into graphite.
7. It form tetrahedral crystals and hybridisation of Carbon-atom is sp³.
8. It has high mp & density.
9. Black diamonds called carbonado contains traces of graphite.
Graphite (Plumbago or black lead)
1. It is soft, greasy, dark greyish colored crystalline solid.
2. It is good conductor of heat and electricity.
3. Its specific gravity is 2.3
4. The hybridization of carbon in graphite is sp² and it has hexagonal layer structure
5. It is chemically more reactive than diamond
6. Its layer structure is held by weak van der Waal’s force.
7. Graphite is used in making for lining and making electrodes of electric furnances, in making refractory crucibles, in making lead pencils, as a moderator in nuclear reactor as lubricant in machinery, as a reducing agent in steel manufacturing.
Forms of Amorphous carbon obtained by destructive distillation
1. Wood charcoal – Obtained from wood
2. Sugar charcoal – Obtained from cane sugar
3. Bone or animal charcoal – Obtained from animal bones
4. Coke charcoal – Obtained from coal
Hydrocarbons
Compounds of carbon and hydrogen are called hydrocarbons. The branch of chemistry in which we study about hydrocarbon and derivatives of hydrocarbon is known as organic chemistry. First organic compound prepared in laboratory was urea (NH₂CONH₂). The natural source of hydrocarbons is petroleum.
Hydrocarbons are classified as :
1. saturated hydrocarbons
2. unsaturated hydrocarbons
3. aromatic hydrocarbons.
1. Saturated hydrocarbons: The hydrocarbons in which carbon atoms are singly bonded are called saturated hydrocarbons. Saturated hydrocarbons are also called alkanes or paraffins. Alkanes are relatively unreactive under ordinary laboratory conditions. So, alkanes are also called paraffins because paraffins means little reactive.

2. Unsaturated hydrocarbons: The hydrocarbons in which carbon atoms are either doubly or triply bonded are called unsaturated hydrocarbons. Doubly bonded carbon atoms (C=C) hydrocarbons are called alkenes. The general formula of alkene is CnH₂n.

Ethene (Ethylene) is used for natural ripening of fruits. Triply bonded carbon atoms (C = C) containing hydrocarbons are called alkynes. The general formula of alkynes are CnH₂n-2.
Example-Ethyne – (Acetylene)
The molecular formula of ethyne is C2H2 and its structural formula is H—C≡C—H
Acetylene is used for artificial ripening of fruit.
3. Aromatic hydrocarbons: These are homocyclic compounds which contain atleast one benzene ring in which carbon atoms are linked to one another by alternate single and double bonds.
In Greek, aroma stands for sweet smell. Compounds in these classification have pleasant smell. Hence, they are called aromatic compounds.

Isomerism: Two or more compounds having same molecular formula but different physical and chemical properties are called isomers and this phenomenon is called isomerism
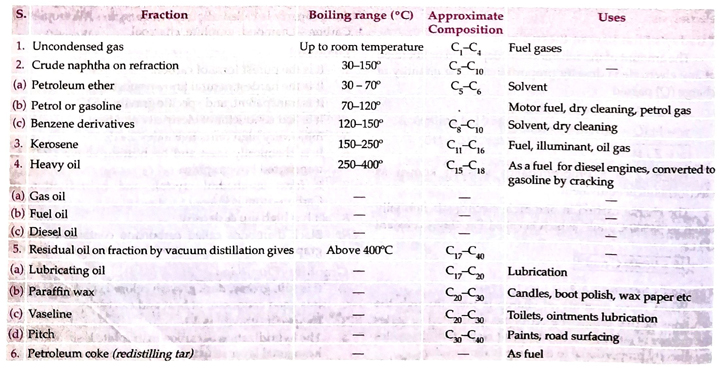
Petroleum: The term petroleum (Latin petra rock, oleum = oil) is applied to the dark-coloured oily liquid with offensive odour found at various depths in many regions below the surface of the earth. It is also called rock oil, mineral oil or crude oil.
A complete list of petroleum products, approximate composition, boiling range and their uses is given ahead.
Follow on Facebook page – Click Here
Google News join in – Click Here
Read More Asia News – Click Here
Read More Sports News – Click Here
Read More Crypto News – Click Here