JKBOSE 9th Class Science Solutions Chapter 7 Matter In Our Surroundings
JKBOSE 9th Class Science Solutions Chapter 7 Matter In Our Surroundings
JKBOSE 9th Class Science Solutions Chapter 7 Matter In Our Surroundings
Jammu & Kashmir State Board JKBOSE 9th Class Science Solutions
J&K class 9th Science Matter In Our Surroundings Textbook Questions and Answers
BASIS AND BASICS
◆ Matter. It is anything which occupies space, has mass and can be judged by any one or more of the known five physical senses. All matter is made up of a large number of extremely small particles called molecules.
◆ Material. It represents a particular kind of matter.
◆ Material can be homogeneous or heterogeneous.
◆ Homogeneous material. It is a material which has uniform composition throughout. It consists of a single phase.
◆ Heterogeneous material. It is a material which does not have a uniform composition throughout. It consists of two or more phases.
◆ Substance. It is a homogeneous material which is made up of only one kind of atoms or material. (Substance always refers to pure substance).
◆ States of Matter. Based upon its physical state, there are three states of matter, i.e. solid, liquid and gas.
◆ There are two new states of matter. These are plasma and Bose-Einstein condensate (BEC).
◆ Solid. It is that state of matter which has a definite mass, volume and shape.
◆ Liquid. It is that state of matter which has a definite mass and volume but has no definite shape.
◆ Gas. It is that state of matter which has a definite mass but has neither definite shape nor definite volume.
◆ The forces of attraction between the particles are maximum in solids, intermediate in liquids, and maximum in gases.
◆ The vacant spaces between the constituent particles and kinetic energy of particles are minimum in case of solids, intermediate in liquids and maximum in gases.
◆ The arrangement of constituent particles is most ordered in case of solids, in case of liquids the layers can slip over each other while in case of gases, there is no order, particles can move randomly.
◆ The different states of matter are interconverted by changing temperature or pressure the or both.
◆ Melting point. It is the temperature at which a solid changes into liquid state.
◆ Boiling point. It is the temperature at which a liquid changes into vapour under atmospheric pressure. At boiling point, vapour pressure of the liquid is equal to atmospheric pressure.
◆ Boiling is a bulk phenomenon as it involves whole of the liquid.
◆ Evaporation. The slow passing out of molecules of a liquid into gaseous state at a bestemperature below its boiling point.
◆The rate of evaporation depends upon the surface area, temperature, humidity and wind speed.
◆ Evaporation causes cooling.
◆ Boiling is a fast process whereas evaporation is a slow process.
◆ Latent heat of fusion is the amount of heat energy required to change 1 kg of solid into liquid at its melting point.
◆ Latent heat of vaporisation is the amount of heat energy required to change 1 kg of a liquid to gas at atmospheric pressure and at its boiling point.
◆ Sublimation. It is the process due to which a solid directly changes into gaseous state on heating and a gaseous state directly changes into solid state on cooling without changing into liquid state.
◆ Vapour. It is a substance which exists in the gaseous state at a temperature lower than the boiling point of its liquid state.
IMPORTANT TERMS/FACTS TO MEMORISE
⇒ Temperature on Kelvin scale = 273 + Temperature on centigrade scale.
TK = 273 + tºC
⇒ Water freezes at 0ºC or 273 K.
⇒ Water boils at 100ºC or 373 K.
⇒ Unites of Latent heat of fusion are k J / kg or kcal / kg.
⇒ Unites of Latent heat of vaporisation are kJ / kg or kcal / kg.
⇒ F – 32 / 9 = C / 5 , F = Temperature on Fahrenhiet scale.
C = Temperature on Centigrade scale.

TEXTBOOK QUESTIONS (SOLVED)
Q. 1. Which of the following are matter ?
Chair, air, love, smell, hate, almonds, thought, cold, cold drink, smell of perfume.
Ans.— Chair, air, almonds, cold drink, smell of perfume.
Q. 2. Give reasons for the following observations :
The smell of hot sizzling food reaches you several metres away, but to get the smell from cold food you have to go close.
Ans.— The smell of hot sizzling food reaches us several metres away, but to get the smell from cold food we have to go close. This is because the rate of diffusion of a gas increases with the increase in temperature. In hot food, the rate of diffusion is large but in cold food, the rate of diffusion is slow.
Q. 3. A diver is able to cut through water in a swimming pool, which property of matter does this observation show ?
Ans.— The diver is able to cut through water in a swimming pool because in water the attractive forces between the molecules are small.
Q. 4. What are the characteristics of the particles of matter ?
Ans.— The characteristics of the particles of matter are :
1. The particles of matter have vacant spaces between them.
2. The particles of matter are always in motion.
3. There are attractive forces between the particles of matter.
Q. 5. The mass per unit volume of a substance is called density.
(density = mass/ volume). Arrange the following in order of increasing density-air, exhaust from chimneys, honey, water, chalk, cotton and iron.
Ans.— The increasing order of density is
Exhaust from chimnies < air < cotton < chalk < honey < iron.
Q. 6 (a) Tabulate the differences in the characteristics of states of matter.
(b) Comment upon the following :
rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy and density.
Ans.— (a) Differences in the characteristics of states of matter :
| Characteristics | Solid | Liquid | Gas |
| Vacant space | Very small | Small | very large |
| Attractive forces between particles | Very large | Small | Negligible |
| Compressibility | Negligible | Low | High |
| Rigidity | High | Low | Now rigid |
| Density | High | Low | Very Low |
| Energy | Small | Large as compared to solid | Very high |
(b) Rigidity. It is the property by virtue of which a substance can retain its shape when a force is applied to it. Solids possess the property of rigidity.
Compressibility. The property by virtue of which the volume of a substance can be decreased by applying force or pressure on it. Gases have high compressibility because of large vacant spaces between the molecules of a gas.
Fluidity. It is the property by virtue of which the molecules of one substance can flow from one point to another. Liquids and gases possess fluidity.
Filling a gas container. A gas fills the container because there are negligible attractive forces between the molecules of a gas and the molecules of a gas move with very high velocities in all possible directions.
Shape. It is the definite geometrical arrangement of constituent particles of a substance. Solids have definite shapes.
Kinetic energy. It is the energy possessed by a particle due to its motion.
Density. Density of a substance is the mass per unit volume. Its units are g/cc or kg/m³.
Q. 7. Give reasons :
(a) A gas fills completely the vessel in which it is kept.
(b) A gas exerts pressure on the walls of the container.
(c) A wooden table should be called a solid.
(d) We can easily move our hand in air but to do the same in solid block of wood we need a Karate expert.
Ans.— (a) This is because in a gas the attractive forces between the particles are negligible and molecules of a gas move with very high speeds in all possible directions.
(b) This is due to the hits or bombardments of the molecules of a gas against the walls of a container.
(c) This is because it has a definite mass, volume and shape.
(d) This is because in air, the attractive forces between the particles are negligible but in a solid block of wood, there are large attractive forces between the constituent particles.
Q. 8. Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why.
Ans.— Ice floats over water because ice has lower density than liquid water. This is because in ice for a given mass volume is more as compared to in liquid water.
Q. 9. Convert the following temperature to celsius scale :
(a) 300 K (b) 573 K ?
Ans.— (a) 300 K = 300 – 273 = 27°C.
(b) 573 K = 573 – 273 = 300°C.
Q. 10. What is the physical state of water at :
(a) 250°C (b) 100°C ?
Ans.— (a) Gas (b) Gas
Q. 11. For any substance, why does the temperature remain constant during the change of state ?
Ans.— This is because the heat supplied is used to overcome the attractive forces between the particles i.e., there is change in potential energy.
Q. 12. Suggest a method to liquefy atmospheric gases.
Ans.— The atmospheric gases can be liquefied by cooling under pressure.
Q. 13. Why does a desert cooler cool better on a hot dry day ?
Ans.— This is because on a hot dry day, due to increase in temperature and lesser humidity the evaporation of water will be faster.
Q. 14. How does the water kept in an earthen pot (matka) become cool during summer ?
Ans.— This is because the water comes out from the pores of earthen pot (matka) and it evaporates. Due to evaporation it causes cooling.
Q.15. Why does our palm feel cold when we put some acetone or petrol or perfume on it ?
Ans.— This is because the particles of acetone or petrol or perfume gain energy from our palm and surroundings and evaporate causing cooling.
Q. 16. Why are we able to sip hot tea or milk faster from saucer rather than a cup ?
Ans.— Because a saucer provide more surface area than cup for evaporation of liquid into vapour and it causes more cooling effect.
Q.17. What type of clothes should we wear in summer ?
Ans.— White cotton clothes.
TEXTBOOK EXERCISES (SOLVED)
Q. 1. Convert the following temperature to the Celsius scale :
(a) 293 K (b) 470 K.
Ans.— (a) 293 K = 293 – 273 = 20°C
(b) 470 K= 470 – 273 = 197°C
Q. 2. Convert the following temperatures to the Kelvin scale :
(a) 25°C (b) 373°C.
Ans.— (a) 25°C = 25 + 273 = 298 K
(b) 373°C = 373 + 273 = 646 K
Q. 3. Give reason for the following observations :
(a) Naphthalene balls disappear with time without leaving any solid.
(b) We can get the smell of perfume sitting several metres away.
Ans.— (a) This is because naphthalene sublimes i.e. it directly changes into vapour without melting.
(b) This is because the molecules of perfume are moving with very high velocities (i.e. diffusion) in all the directions.
Q. 4. Arrange the following substances in increasing order of forces of attraction between the particles-water, sugar, oxygen.
Ans.— The increasing order of intermolecular forces of attraction is Oxygen < Water < Sugar.
Q. 5. What is the physical state of water at :
(a) 25°C (b) 0°C 62 (c) 100°C?
Ans.— (a) At 25° C, water is liquid.
(b) At 0° C, water is solid (Ice).
(c) At 100° C, water is gas (Steam).
Q. 6. Give two reasons to justify that :
(a) Water at room temperature is a liquid.
(b) An iron almirah is a solid at room temperature.
Ans.— (a) At room temperature water is liquid because the attractive forces between the molecules of water are small and can move from one point to another.
(b) An iron almirah is solid because the molecules are held together by strong intermolecular attractive forces and the molecules or particles are very close to each other.
Q. 7. Why is ice at 273 K more effective in cooling than water at the same temperature ?
Ans.— Ice at 273 K is more effective in cooling than water at 273 K, this is because in ice, the molecules have lower energy as compared to the particles in the liquid water at the same temperature (273 K) and require latent heat of fusion for melting.
Q. 8. What produces more severe burns: boiling water or steam ?
Ans.— Steam produces severe burns as compared to boiling water. This is because in steam the molecules have higher kinetic energies than in boiling water.
Q. 9. Name A, B, C, D, E and F in the following diagram showing state change–
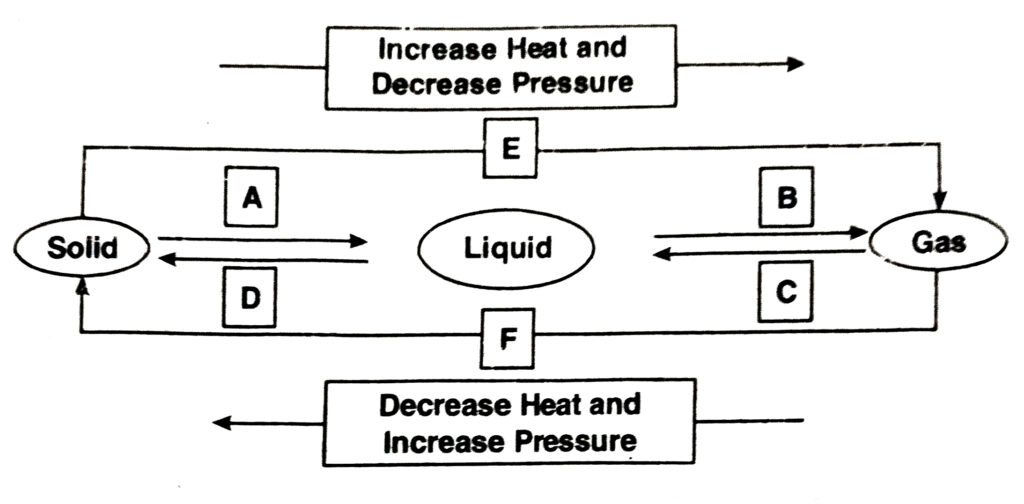
Ans.—

A— Fusion
B— Vaporisation
C— Condensation
D— Solidification
E— Sublimation
F— Sublimation
ADDITIONAL IMPORTANT QUESTIONS
LONG ANSWER TYPE QUESTIONS
Q. 1. How will you explain the three states of matter on the basis of Kinetic Model ?
Ans.— Solid. In solids the constituent particles are closely packed and there are attractive forces between them. Therefore, the particles have low kinetic energies and can’t move from one position to another but they have only vibratory motion. Hence solids have definite shapes and definite volumes, e.g. wood, rock, copper, iron, cement, stone etc.
Liquid. In liquids the molecules have higher kinetic energies, distances between the molecules are more and attractive forces between the molecules are small. Therefore, the molecules of a liquid can move from one position to the another within the liquid. Hence the liquid can take the shape of the container. But the volume of the liquid remains fixed because the molecules can’t leave the liquid, e.g. alcohol, milk, benzene, kerosene oil, petrol etc.
Gas. In the gases, the molecules have high kinetic energies, there are large vacant spaces between the molecules and there are negligible attractive forces between the molecules. Therefore, in the gases molecules can move randomly and occupy the whole space available to them. Hence, they don’t have definite volumes and shapes, e.g. hydrogen, oxygen, nitrogen, sulphur dioxide, carbon dioxide etc.
Q. 2. Define the terms given below and answer the questions associated with them.
(a) Sublimation : Which of the following substances sublime ?
Ice, mercury, dry ice, iodine.
(b) Solid: Why do not solids diffuse in one another ?
(c) Liquid: Why do liquids flow?
(d) Freezing point: What is the freezing point of water ?
(e) Gas: Why are gases compressible and show diffusion ?
Ans.— (a) Sublimation. It is the process in which a solid directly changes into vapour on heating and on cooling the gas directly changes into solid.
Dry ice and iodine sublime.
(b) Solid. It is that state of matter which has definite mass, volume and shape. e.g. sugar, rubber, table, stone etc.
The solids don’t diffuse because in the solid state the vacant spaces between the molecules are very small and molecules of a solid can’t move from one position to another.
(c) Liquid. A liquid is that state of matter which has definite mass and volume but has no definite shape. e.g. milk, petrol, kerosene oil, water etc.
Liquids flow because the attractive forces between the molecules of a liquid small and the molecules can freely move within the liquid.
(d) Freezing point. It is the temperature at which a liquid changes into a solid state by giving out heat energy. For examples freezing point of water is 0°C.
(e) Gas. It is defined as that state of matter which has definite mass only but has neither definite shape nor definite volume. For example, carbon dioxide, oxygen, hydrogen, ammonia etc.
The gases are compressible and show diffusion because there are large vacant space between the molecules of a gas and molecules of a gas are always in motion.
Q. 3. Complete the following with suitable words :
1. There are ……………… states of matter.
2. In gases there are……………….vacant spaces between the molecules.
3. Evaporation causes……………… .
4. Gases changes into liquid on ……………
temperature and ……………….. pressure.
5. Liquids have ……… free surfaces.
Ans.— 1. Three 2. large 3. cooling 4. decreasing, increasing 5. one.
Q. 4. State whether the following statements are true or false :
(a) The freezing point of water is 0°C.
(b) A decrease in pressure lowers the boiling point of liquid.
(c) During sublimation, the substance must be heated.
(d) During interconversion of matter, mass remains constant.
(e) Intermolecular forces in case of solids are maximum.
Ans.— (a) True (b) True (c) False (d) True (e) True.
Q. 5. What do you understand by interconversion of different states of matter and how are these changes brought about ?
Ans.— The process of change of matter from one state to another is called interconversion of states of matter. The states of matter can be changed by changing temperature and pressure.
1. By changing temperature. By increasing temperature, the solid can be changed into liquid and liquid can be changed into gas. This is because on increasing temperature, the kinetic energies of the molecules increase and they can move freely. The reverse changes occur on cooling.
For example, ice changes into liquid water at 0°C and water changes into steam at 100°C (under normal pressure).
2. By changing pressure. When the pressure is lowered the liquid starts boiling at a lower temperature and changes into gaseous state. Similarly, by applying high pressure, a gas can be liquefied.
For example, carbon dioxide can be liquefied by applying a pressure of 70 atmosphere under normal temperature.
SHORT ANSWER TYPE QUESTIONS
Q. 1. Heat, light, shadow, love, radio waves are not considered as matter, why ?
Ans.— This is because these are massless and do not occupy space.
Q. 2. What are the two new states of matter in addition to solid, liquid and gaseous states ?
Ans.— These are :
1. Plasma. It is produced in stars at very high temperature.
2. Bose-Einstein condensate. It is formed by cooling a gas of extremely low density to super low temperature.
Q. 3. Name five substances which are solids, five which are liquids and five which are gases at room temperature.
Ans.— Examples of solids. Iron, copper, silver, glass and wood.
Examples of liquids. Water, milk, mercury, kerosene oil and petrol.
Examples of gases. Hydrogen, oxygen, carbon dioxide, nitrogen, helium.
Q. 4. What is the difference between gas and vapour ?
Ans.— A gas is a substance which exists in the gaseous state at a temperature equal to or more than the boiling point of its liquid state. e.g. oxygen, hydrogen, nitrogen etc.
A vapour is a substance which exists in the gaseous state such that its temperature is lower than that of boiling point of its liquid state e.g. water vapour, iodine vapour etc.
Q. 5. Rubber band can change its shape, is it a solid ?
Ans.— Rubber band is a solid because it changes its shape under force and return to its original state when the applied force is removed.
Q. 6. What happens when the vacant spaces between the particles of a liquid decreases? How is this possible ?
Ans.—The liquid will change into solid. This is carried out by decreasing the temperature or cooling the liquid.
Q. 7. Why do we observe water droplets on the outer surface of a glass containing ice cold water ?
Ans.— This is because water vapour present in air, on coming in contact with glass containing ice-cold water lose energy and get converted into liquid state which appear as water droplets.
Q. 8. Why should we wear cotton clothes in summer ?
Ans.— This is because in summer, we perspire more and cotton being a good absorber of water, absorbs the sweat and exposes it to the atmosphere for easy evaporation and evaporation causes cooling by absorbing heat from the surroundings or body surface.
Q. 9. State your observations in the following cases :
(a) Ammonium chloride is heated in a hard glass test tube.
(b) Carbon dioxide is compressed to 70 times the atmospheric pressure.
Ans.— (a) When ammonium chloride is heated in a hard glass test tube, dense white fumes of ammonium chloride fill the tube and deposit to give a white powder near the mouth of the tube (cooler parts).
(b) When carbon dioxide is compressed to 70 atmospheric pressure, it changes into solid state.
Q. 10. Why is sponge solid although it can be compressed ?
Ans.— Th is because sponge has minute holes in which there is entraped air. When it is pressed, the air is expelled out.
Q. 11. Why does a gas fill the container completely ?
Ans.— The molecules in a gas are far apart and are in a state of rapid random motion in all possible directions with very high speeds. They move throughout the container in which they are put. Thus, a gas fills the container completely.
Q. 12. What are the uses of interconversion of matter ?
Ans.— These are :
1. Water is converted into steam by heating. Steam is used to run turbines and to generate electricity.
2. Metals are melted by heating and these molten metals can be converted into alloys and cast into machinery parts.
3. Water can be changed into steam as well as ice. Therefore, in nature, ice (or snow), liquid water and water vapour are available.
Q. 13. Give two differences between boiling and evaporation.
Ans.—
| Boiling | Evaporation |
|
It takes place at a fixed temperature called boiling point of the liquid.
|
It takes place at all temperatures. |
| It is a fast process. | It is a slow process |
Q. 14. List the factors which effect evaporation.
Ans.— There are :
1. Exposed surface area
2. Temperature
3. Humidity in the air
4. Wind speed.
Q. 15. Give an experiment to prove that gases are more compressible as compared to liquids.
Ans.— Take a 100 ml syringe and close its nozzle by inserting it in a rubber cork or stopper. Remove the piston from the syringe so that whole of syringe is filled up with air. Apply a little vaseline to the piston and insert the piston. Compress piston and it moves downwards easily. Hence air is compressed to m a very small volume.

Now fill the syringe with water and repeat the above experiment. The piston moves hardly downwards, indicating that there is a negligible compression in volume.
The above experiment indicates that gases are highly compressible as compared to liquids.
Q. 16. How do aquatic plants and animals survive ?
Ans.— The gases from the atmosphere diffuse and dissolve in water. There gases mainly contain carbon dioxide and oxygen. These are taken up for their by the aquatic plants and animals survival.
Q. 17. State what is observed when iodine is heated in a test tube ?
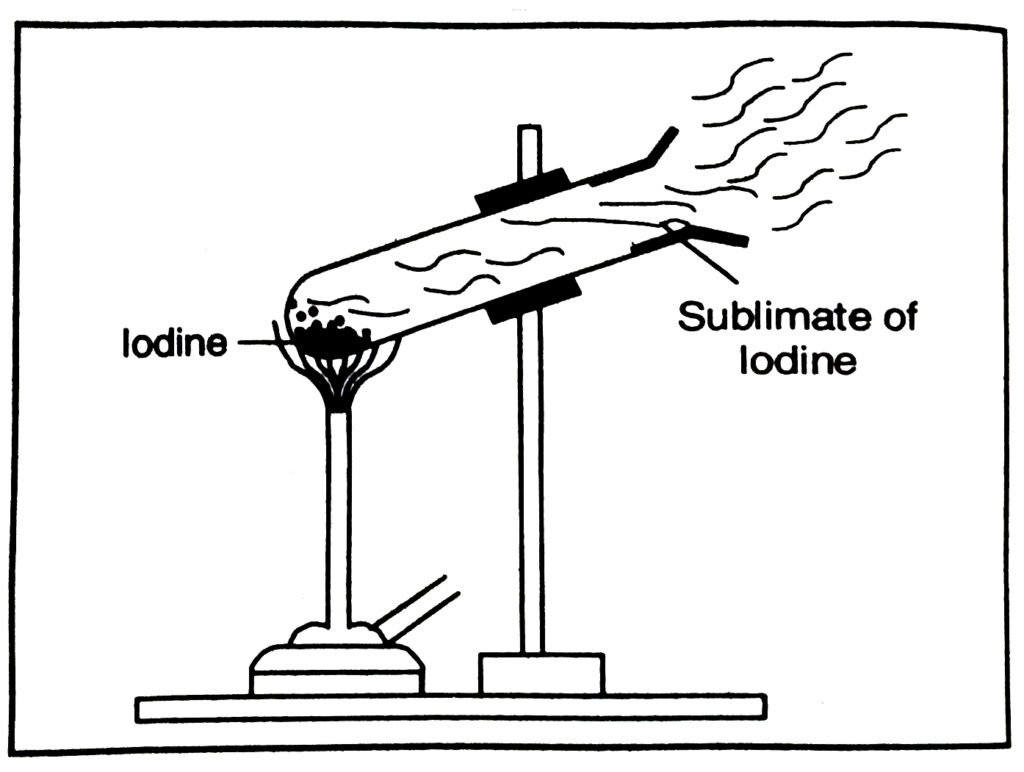
Ans.— When some crystals of iodine are heated slowly in a test tube, the shiny grey iodine changes directly into vapour without melting. The vapour deposit as grey particles called sublimate on the upper cooler parts of the tube.
Q. 18. Arrange the following in the increasing order of density :
air, iron, honey, water
Ans.— Air < Water < Honey < Iron (Increasing order of Density)
Q. 19. Which phenomenon occurs during the following changes :
(i) Size of naphthalene balls decreases
(ii) Wax melts in the sun
(iii) Drying of wet clothes
(iv) Formation of clouds.
Ans.— (i) Sublimation
(ii) Fusion
(iii) Evaporation
(iv) Condensation.
Q. 20. Fill in the blanks :
(i) A vapour on cooling changes into ……….and on further cooling changes into……………. .
(ii) Matter changes from one state to another either by raising the……….or lowering the……….. .
(iii) A change in which a solid, on heating, directly changes into……………state and the……………..on cooling again changes into…………state is calle..…………….
(iv) The intermolecular spaces are ……..in gaseous and …………in solids.
Ans.— (i) liquid, solid. (ii) temperature, temperature. (iii) gaseous, gas, solid, sublimation. (iv) minimum, maximum.
VERY SHORT ANSWER TYPE QUESTIONS
Q. 1. Give four examples of solids which sublime on heating.
Ans.— Iodine, ammonium chloride, camphor, naphthalene.
Q. 2. Explain why solids can’t be compressed.
Ans.— This is because in the solids, the particles are closely packed and the vacant spaces between them are negligible.
Q. 3. In which of the following substances the intermolecular forces are (a) weakest (b) strongest ? Water, alcohol, sugar, sodium chloride, carbon dioxide gas.
Ans.— (a) Carbon dioxide gas.
(b) Sodium chloride.
Q. 4. What is common between three states of matter ?
Ans.— The three states of matter occupy space and have mass.
Q. 5. Give two main characteristics used to distinguish between three states of matter.
Ans.— 1. Fluidity 2. Compressibility.
Q. 6. Why does a gas exert pressure ?
Ans.— This is due to the bombardment of the molecules of a gas against the walls of the container.
Q. 7. Why do liquids flow ?
Ans.— This is because there are some vacant spaces between the particles and less attractive forces between the particles.
Q. 8. Gases have more compressibility as compared to liquids, why ?
Ans.— This is because in gases the vacant spaces between the particles are very large as compared to that in liquids.
Q. 9. Light and sound are not considered to be matter, why ?
Ans.— Sound is not considered to be matter because it has no mass and does not occupy space.
Light has dual character. Sometime it behaves as matter and sometime behaves as wave.
Q. 10. Why does the temperature not rise during the process of melting and boiling, though heat energy is constantly supplied ?
Ans.— This is because during the process of melting and boiling the heat energy supplied is absorbed by the molecules to increase potential energy and to decrease the attractive forces between the molecules.
MULTIPLE CHOICE QUESTIONS
Select the Correct Answer :
1. Which of the following characteristics are associated with solid state ?
(A) Definite shape, compressible, fixed volume.
(B) Definite shape, highly compressible, fixed volume.
(C) No definite shape, highly compressible, closely packed.
(D) Definite shape, incompressible, closely packed.
Ans.— (D) Definite shape, incompressible, closely packed.
2. The physical state of water at 20°C is :
(A) Solid
(B) Liquid
(C) Gas
(D) May be solid or liquid.
Ans.— (B) Liquid
3. The state of matter which is highly compressible :
(A) Solid
(B) Liquid
(C) Gas
(D) None.
Ans.— (C) Gas
4. Which is a slow process ?
(A) Buring of saw dust
(B) Burning of log of wood
(C) Burning of Coal pieces
(D) All are fast processes.
Ans.—(A) Buring of saw dust
5. Which of the following is not a matter ?
(A) Air
(B) Smell
(C) Chair
(D) Cold drink.
Ans.— (A) Air
6. The intermolecular attractive forces are maximum in :
(A) Solids
(B) Liquids
(C) Gases
(D) None.
Ans.— (A) Solids
7. The constituent particles have only vibratory motion in :
(A) Solids
(B) Liquids
(C) Gases
(D) All.
Ans.— (A) Solids
8. The state of matter which is least compressible :
(A) Solid
(A) Solid
(C) Gas
(D) All are equally compressible.
Ans.— (A) Solid
9. 300 K is equal to :
(A) 0°C
(B) 27°C
(C) 300°C
(D) -27°C.
Ans.— (B) 27°C
Follow on Facebook page – Click Here
Google News join in – Click Here
Read More Asia News – Click Here
Read More Sports News – Click Here
Read More Crypto News – Click Here