PSEB Solutions for Class 9 Science Chapter 4 Structure of the Atom
PSEB Solutions for Class 9 Science Chapter 4 Structure of the Atom
PSEB 9th Class Science Solutions Chapter 4 Structure of the Atom
→ It was known by 1900 that the atom was not a simple, indivisible particle but contains at least one sub-atomic particle i.e., electron identified by J.J. Thomson.
→ Even before the electron was identified by J.J. Thomson, E. Goldstein in 1886 discovered the presence of new radiations in a discharge tube called canal rays.
→ The canal rays were positively charged radiations which led to the discovery of the proton.
→ Proton has a charge, equal in magnitude but opposite in sign to that of electron and a mass approximately 2000 times as that of the electron.
→ Generally, the electron is represented by ‘e’ and a proton as ‘p’.
→ The mass of a proton is taken as one unit and charge +1.
→ The mass of an electron is considered to be negligible and its charge -1.
→ E. Rutherford discovered the nucleus of an atom on the basis of an α-ray scattering experiment.
→ E. Rutherford was awarded the Nobel prize in chemistry for his famous work discovery of radioactivity and the discovery of the nucleus of the atom.
→ On the basis of his experiment, Rutherford put forward the nuclear model of an atom.
→ According to Rutherford’s model, there is a positively charged centre of an atom called a nucleus and contains nearly all the mass and all the positive charge.
→ The electrons revolve around the nucleus in circular paths. The size of the nucleus is very small as compared to the size of the atom.
→ Neils Bohr’s model of the atom was more successful.
→ He suggested that only certain special orbits known as discrete orbits of electrons are allowed inside the atom. While revolving electrons do not radiate energy.
→ J. Chadwick discovered another sub-atomic particle that had no charge but mass nearly equal to a proton. This particle is called the neutron.
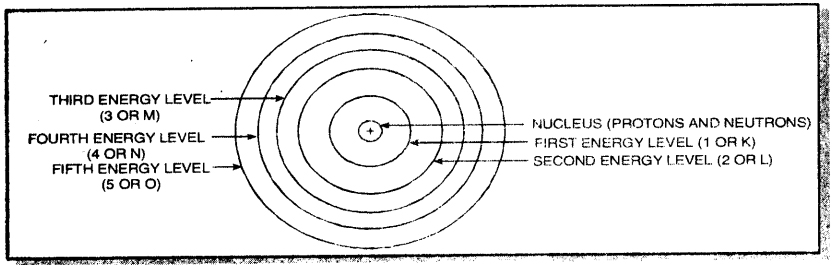
→ The orbits or shells in an atom are designated as K, L, M, N ………… shells starting from the nucleus side.
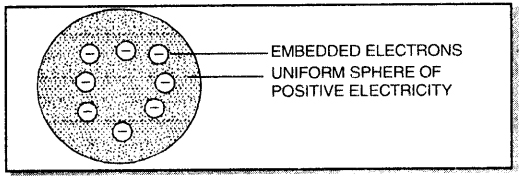
→ J.J. Thomson suggested that an atom is a uniform sphere of positive electricity in which electrons are embedded it.
→ The total positive charge is equal to the total negative charge and the atom, on the whole, is electrically neutral.
→ Electron is a negatively charged particle with 1.602 × 10-19 coulomb negative charge (-1 unit) and mass 9.1089 × 10-19 kg (negligible mass). It is represented by the symbol ‘e’. It is a fundamental particle of an atom.
→ Proton is a positively charged particle with a 1.602 × 10-19 coulomb positive charge (+1 unit) and mass 1.672 × 10-27 kg, it is represented by the symbol ‘p’. It is a fundamental particle of an atom.
→ Neutron is a neutral particle with no charge and mass equal to 1.678 × 10-27 kg. It is represented by the symbol ‘n’. It is a fundamental particle of an atom.
→ The nucleus is the small, positively charged, and heavy central portion in an atom that contains in it protons and neutrons.
→ Nucleons. The neutrons and protons present in the nucleus of an atom are collectively known as nucleons.
→ An atomic number of an element represents the number of protons in an atom. It is denoted by the symbol Z.
→ Shells of an atom are designated as K, L, M, N, etc. These are also called energy levels.
→ The valence shell of an atom represents the outermost shell where electrons are present and the electrons are called valence electrons.
→ A mass number of an element is the sum of the number of protons and neutrons present in the nucleus of the atom. It is denoted by A.
→ The valence shell of an atom represents the outermost shell where electrons are present and the electrons are called valence electrons.
→ Valency. It is the combining capacity of an atom of the element.
→ Isotopes are the atoms of the same element having the same atomic number but different mass numbers.
→ Isobars are the atoms of the different elements having the same mass number but different atomic numbers.
PSEB 9th Class Science Important Questions Chapter 4 Structure of the Atom
Long Answer Type Questions:
Question 1.
What is Rutherford’s ∝-ray scattering experiment? Give its observations. How does Rutherford explain this observation?
Answer:
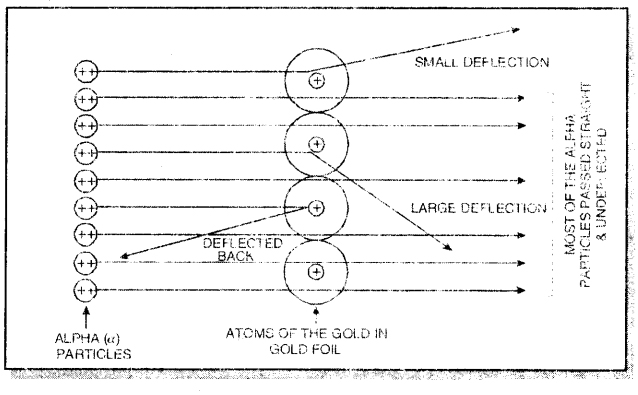
Rutherford’s ∝-ray scattering experiment: In this experiment, Rutherford obtained a thin stream of fast-moving alpha (∝) particles from radioactive source (P0) and was allowed to strike against a thin foil of heavy metal like gold. Alpha particles are also called helium nuclei (He2+ with + 2 unit charge and 4 a.m.u. mass). As a result of the experiment, he made the following observations:
- Most of the ∝-particles passed through the gold foil without any deflection from their original path.
- Some of the ∝-particles got deflected by small angles from their original path.
- A few ∝-particles (approximately 1 out of 10,000) were deflected to a large extent. In some cases, the a-particles came back in the same direction after striking the gold foil.
Explanation for the observations:
We know that the gold foil is made up of atoms of gold which are placed side by side. Since most of the ∝-particles passed through these atoms undeflected they did not come across any obstruction in their path, this shows that most of the space in the atom is light or hollow.
The electrons with negligible mass were supposed to be present in this portion called extra nuclear portion. The a-particies were deflected from their path and a very few came back in the same direction. This shows that they must have met with some obstruction in their path.
The obstruction must be
1. Heavy because a-particles are high speed particles and can remove light obstruction from their path.
2. Small because only a few a-particles got deflected.
3. Positively charged because some a-particles were repelled by this obstruction.
This small, heavy and positively charged portion inside an atom was called nucleus. The protons with mass and positive charge were supposed to be present in the nucleus. Later on, neutrons were also found to be present in the nucleus of the atom.
Question 2.
Discuss in brief Rutherford’s Model of atom.
Answer:
The main points of Rutherford’s model of atom are listed as follows:
1. An atom has two parts. These are nucleus and extra nuclear portion.
2. The nucleus contains protons while electrons are present in the extra nuclear portion.
3. Most of the mass of the atom is due to the nucleus because electrons have negligible mass.
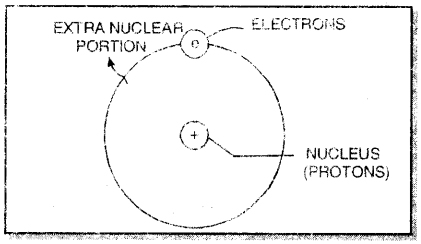
4. The volume occupied by the nucleus is very small as compared to the volume occupied by extra nuclear portion.
5. The electrons in the extra nuclear portion are not stationary. These are revolving around the nucleus. The electrons are called planetary electrons because they can be compared to various planets which revolve around the sun. The nucleus may be compared with the sun.
Question 3.
Discuss the arrangement of the electrons in the different energy levels.
Or
Describe Bohr-Bury scheme for the distribution of electrons in the various orbits or energy levels of an atom.
Answer:
The distribution of electrons in the energy levels is given with the help of Bohr Bury scheme. It is given as follows:
1. The energy levels or energy shells are filled in order of increasing energies. The electrons first enter the K shell (n = 1. which is closest to the nucleus. This is followed by L shell (n = 2), M shell (n = 3) and so on. Here V represents the number of the shell.
2. The maximum number of electrons in any shell is given as 2n2 (n = number of the shell). The distribution in the first four energy shells is given in the table given below:
Distribution of electrons in first four energy shells according to 2n2 rule.
| Symbol of shell | Number of shell (n) | Maximum number of electrons (2n2) |
| K | 1 | 2 x (1)2 = 2 |
| L | 2 | 2 x (2)2 = 8 |
| M | 3 | 2 x (3)2 = 18 |
| N | 4 | 2 x (4)2 = 32 |
3. The outermost energy shell cannot have more than 8 electrons. The next inner shell called penultimate shell, cannot have more than 18 electrons.
4. It is not necessary that an energy level or shell is fully filled before the filling in the next energy level starts. In fact, filling of electrons in a new shell starts when any shell contains 8 electrons.
On the basis of the above scheme, let us discus with mass number 24 and atomic number 12.
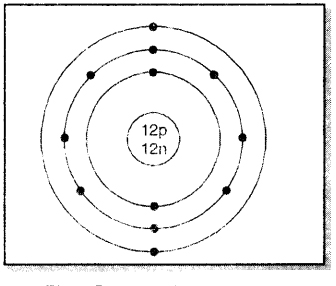
- Mass no. of atom (A) = 24
- Atomic no. of atom (Z) = 12
- No. of protons in nucleus = 12
- No. of neutrons in nucleus = 12
- No. of electrons to be distributed = 12
- The distribution in the various energy shells: K L M: 2, 8, 2
Question 4.
Give the electronic distribution in the first twenty elements on the basis of Bohr-Bury scheme.
Answer:
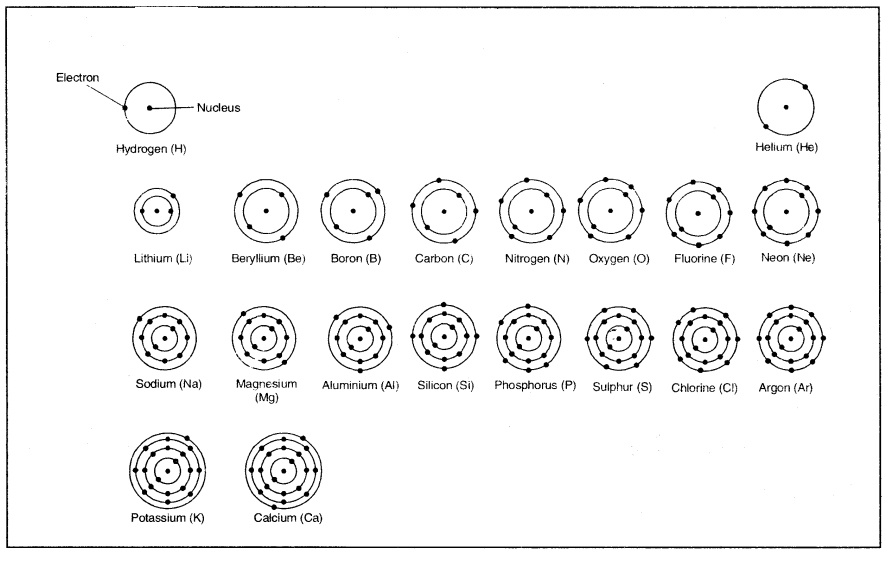
On the basis of Bohr-Bury scheme, the distribution of electrons in first twenty elements (Z = 1 to Z = 20) is given below:
Table: Electronic distribution in atoms of first 20 elements
| Element (Symbol) | Mass No. (A) | Atomic No. (Z) | No. of Protons (P) | No. of Electrons (e) | No. of Neutrons (n) (A-Z) | Electronic Distribution | |||
| K | L | M | N | ||||||
| 1. Hydrogen (H) | 1 | 1 | 1 | 1 | 0 | 1 | |||
| 2. Helium (He) | 4 | 2 | 2 | 2 | 2 | 2 | |||
| 3. Lithium (Li) | 7 | 3 | 3 | 3 | 4 | 9 | 1 | ||
| 4. Beryllium (Be) | 9 | 4 | 4 | 4 | 5 | 2 | 2 | ||
| 5. Boron (B) | 11 | 5 | 5 | 5 | 6 | 2 | 3 | ||
| 6. Carbon (C) | 12 | 6 | 6 | 6 | 6 | 2 | 4 | ||
| 7. Nitrogen (N) | 14 | 7 | 7 | 7 | 7 | 2 | 5 | ||
| 8. Oxygen (O) | 16 | 8 | 8 | 8 | 8 | 2 | 6 | ||
| 9. Fluorine (F) | 19 | 9 | 9 | 9 | 10 | 2 | 7 | ||
| 10. Neon (Ne) | 20 | 10 | 10 | 10 | 10 | 2 | 8 | ||
| 11. Sodium (Na) | 23 | 11 | 11 | 11 | 12 | 2 | 8 | 1 | |
| 12. Magnesium (Mg) | 24 | 12 | 12 | 12 | 12 | 9 | 8 | 2 | |
| 13. Aluminium (Al) | 27 | 13 | 13 | 13 | 14 ‘ | 2 | 8 | 3 | |
| 14. Silicon (Si) | 28 | 14 | 14 | 14 | 14 | 2 | 8 | 4 | |
| 15. Phosphorus (P) | 31 | 15 | 15 | 15 | 16 | 2 | 8 | 5 | |
| 16. Sulphur (S) | 32 | 16 | 16 | 16 | 16 | 2 | 8 | 6 | |
| 17. Chlorine (Cl) | 35 | 17 | 17 | 17 | 18 | 2 | 8 | 7 | |
| 18. Argon (Ar) | 40 | 18 | 18 | 18 | 22 | 2 | 8 | 8 | |
| 19. Potassium (K) | 39 | 19 | 19 | 19 | 20 | 2 | 8 | 8 | 1 |
| 20. Calcium (Ca) | 40 | 20 | 20 | 20 | 20 | 2 | 8 | 8 | 2 |
Special cases of Potassium and Calcium:
- In case of Potassium (Z = 19), the M shell has only 8 electrons and not 18. Actually, when the M shell gets 8 electrons, the filling of electrons starts in the next shell which is N-shell. When the N-shell gets 2 electrons, the electron are again- filled in the M shell.
- Thus, electronic distribution in potassium (Z = 19) is
K L M N
2 8 8 1 (M shell has 8 electrons and N shell has 1 electron) - Similarly, the electronic distribution in calcium (Z = 20) is:
K L M N: 2 8 8 2 (M shell has 8 electrons and N shell has 2 electrons)
Question 5.
Define the terms:
- Atomic number
- Mass number
- Atom
- Ion
- Element
- Orbit
- Nucleons
Answer:
- Atomic number: It is the number of protons present in the nucleus of an atom of the element.
- Mass number: It is the sum of number of protons and neutrons present in the nucleus of an atom of the elements.
- Atom: It is the smallest unit of matter which takes part in chemical reactions and it may or may not exist independently.
- Ion: It is an atom or group of atoms having charge positive or negative and can exist freely in solution.
- Element: It is a pure substance which can neither be built up nor broken into two or more still simpler substance by any physical or chemical method and it consists of only one kind of atoms.
- Orbit: It is the fixed circular path traced by an electron revolving around the
- Nucleons: The protons and neutrons present in the nucleus of an atom of the element are collectively known as nucleons.u
Short Answer Type Questions:
Question 1.
What do you understand by the structure of atom?
Answer:
According to the Dalton’s atomic theory, the smallest particle of matter is called atom. But later studies have shown that an atom is further made up of sub-atomic particles called electrons, protons and neutrons. Different atoms have different number of such particles and they also differ in the arrangement of these particles. Therefore, the atoms have different properties.
Question 2.
What happens when electric field is applied to the path of cathode rays? What does it show?
Answer:
When electric field is applied to the path of the cathode rays, these are deflected towards the positive plate of the field. This shows that the cathode rays consist of negatively charged particles.
Question 3.
What is the information conveyed by the following observations :
- Atom is electrically neutral,
- Mass of the atom is due to the nucleus.
Answer:
- If an atom is electrically neutral, this means that it will have equal number of protons and electrons. Both protons and electrons have 1 unit charge but with opposite sign.
- This means that nucleus contains in it both protons and neutrons which have mass.
Question 4.
What is the significance of number of protons found in the atoms in each of the different elements?
Answer:
The number of electrons in atom of the element is equal to the number of protons. By knowing no. of protons, we can calculate no. of electrons and hence its properties.
Question 5.
Write the electronic configuration of the elements A, B, C, D, E with atomic numbers 5, 6, 14, 13 and 15. Which have similar chemical properties?
Answer:
| Element | Atomic No. (Z) | Electronic configuration | ||
| K | L | M | ||
| A | 5 | 2 | 3 | |
| B | 6 | 2 | 4 | |
| C | 14 | 2 | 8 | 4 |
| D | 13 | ? | 8 | 3 |
| E | 15 | 2 | 8 | 5 |
Elements B and C. have similar chemical properties because both of them have 4 valence elt, trons.
Elements A and D have similar chemical properties because both of them have 3 valence electrons.
Question 6.
Five species P, Q, R, S, T have electrons, protons and neutrons are as follows:
| Species | Electrons | Protons | Neutrons |
| P | 4 | 3 | 4 |
| Q | 8 | 9 | 9 |
| R | 17 | 17 | 20 |
| S | 17 | 17 | 18 |
| T | 18 | 18 | 22 |
Find
- a cation
- an anion
- a noble gas and
- a pair of isotopes.
Answer:
- Cation – Q
- Anion – P
- A noble gas = T
- Pair of isotopes = R and S (because they have same number of electrons and protons but different number of neutrons).
Question 7.
Helium has only 2 electrons in the K-shell. But it is called an inert gas element. Why?
Answer:
Helium (He) atom has only one shell (K-shell) which can have a maximum of 2 electrons only. It cannot have more than 2 electrons. As a result, the combining capacity of He is zero. Therefore, its valency is also zero.
Question 8.
Give labelled diagram to show how are cathode rays produced?
Answer:
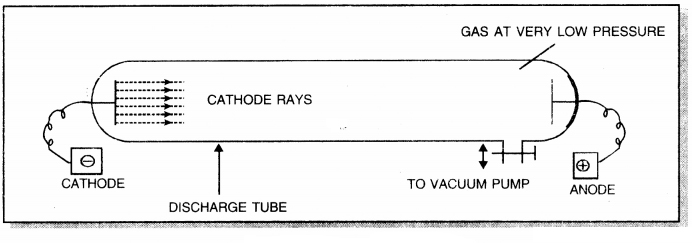
Question 9.
An atom has mass number A and atomic number Z.
1. How many protons are present in the nucleus?
2. How many electrons are revolving around the nucleus?
3. How many neutrons are present in its nucleus?
Answer:
Mass number = A
Atomic number = Z
1. No. of protons present in the nucleus = Z
2. No. of electrons revolving around the nucleus = Z
3. No. of neutrons present in the nucleus = A – Z.
Question 10.
State three ways by which a proton differs from an electron.
Answer:
| Proton | Electron |
| 1. It has one unit of positive charge.
2. It has mass 1.6 x 10-24 g. 3. It is present in the nucleus of an atom. |
It has one unit of negative charge.
It has mass 9.1 x 10″28 g. It is present in the extranuclear part of an atom. |
Question 11.
What are isotopes? Give two uses of isotopes. Name the isotopes of hydrogen. Give their structures.
Answer:
Isotopes. The atoms of the same element having the same atomic number but different mass numbers are called isotopes.
Use of isotopes:
1. Isotopes of hydrogen, oxygen, carbon are used to study the mechanisms of organic reactions.
2. Some radioactive isotopes are used for the treatment of cancer and other diseases. The isotopes of hydrogen are:
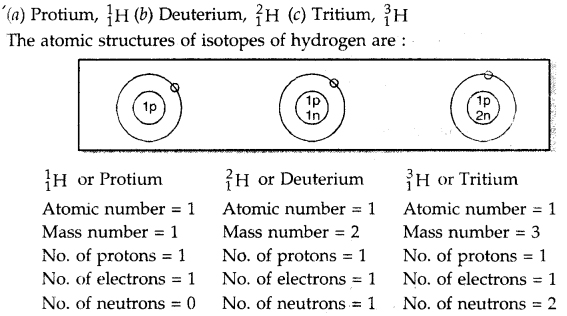
Question 12.
Explain why elements have fractional atomic masses?
Answer:
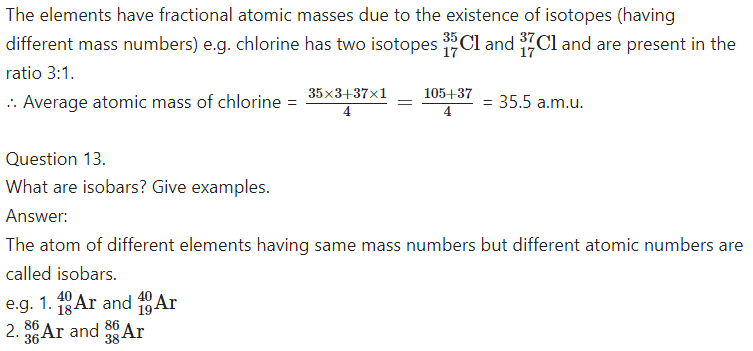
Question 14.
Sulphur has an atomic number 16 and a mass of 32. State the number of protons and neutrons in the nucleus of sulphur. Give a simple diagram to show the arrangement of electrons in an atom of sulphur.
Answer:
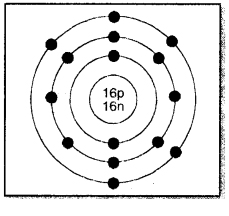
Atomic number of S = 16
Mass number of S = 32
∴ No. of protons = 16
No. of neutrons = 32 – 16 = 16
No. of electrons = 16
Question 15.
Write down the electronic configuration of the following:
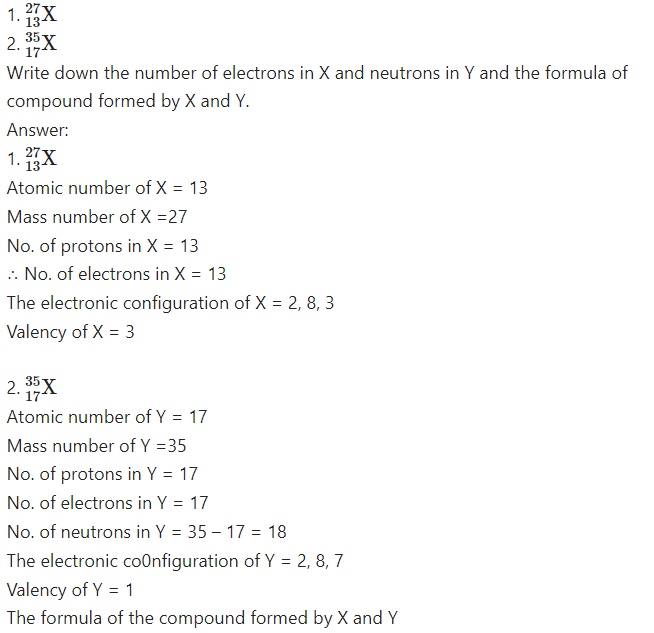
Question 16.
Give the important properties or characteristics of isotopes.
Answer:
- The isotopes of an element have same atomic number (Z) i.e. the same number of electrons and same number of protons. Hence, they have identical chemical properties.
- Isotopes of an element have same number of valence electrons and hence same valency.
- Isotopes of an element have different mass numbers (or different number of neutrons) and hence they have different physical properties such as mass, density, melting point, boiling point etc.
Question 17.
An atom of the element is represented as 11x?
- What does the numeral 23 indicate?
- What does the nemeral 11 indicate?
- What is the number of protons in X?
Answer:
- 23 indicates mass number of X.
- 11 indicates atomic number of X.
- No. of protons in X = Atomic number = 11.
Question 18.
The atom of an element is made up of 4 protons, 5 neutrons and 4 electrons. what are its atomic number and mass number?
Answer:
No. of proton = 4
Atomic number of the element, Z = 4
No. of neutrons = 5
∴ No. of protons + No. of neutrons =4 + 5 = 9
∴ Mass number of the element, A = 9.
Question 19.
Calculate the number of neutrons in the following elements:

Question 20.
Give two conditions under which cathode rays are produced.
Answer:
Cathode rays are produced when a gas is subjected to the action of
1. High voltage of the order of 5000-10000 V and
2. Under a low pressure of the order of 10-3 atm of Hg.
Question 21.
The electronic configuration of P (Z = 15) is 2, 8, 5. Why is the valency of phosphorus 3 and not 5?
Answer:
The electronic configuration of phosphorus (P) shows that it has 5 valence electrons. Its valency is expected to be 5. But it is not possible for the atom to lose 5 electrons present in the valence shell because the energy required to remove these electrons will be very high. Therefore, phosphorus atom has valency equal to 3 because to gain 3 electrons is easier than losing 5 electrons as it requires less energy.
Question 22.
What information is conveyed by the statement that the mass number of magnesium is 24 and the atomic number is 12?
Answer:
The atomic number (12) of magnesium indicates that its atom has 12 protons in the nucleus and an equal number of electrons in the extra nuclear portion. The mass number (24) points out that there are also 12 neutrons (24 – 12 = 12) present in the nucleus alongwith protons.
Question 23.
Is it possible for an atom to have 12 protons and 13 electrons? Explain.
Answer:
No, it is not possible. An atom must always be electrically neutral. This means that it has no net charge present on it. Now, each proton has one unit positive charge and each electron has one unit negative charge. In a neutral atom, the number of electrons and protons must always be the same. Thus, an atom cannot have 12 protons and 13 electrons.
Question 24.
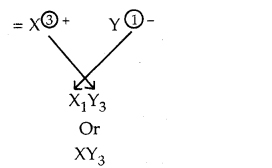
Give the important isotopes of hydrogen, carbon, oxygen and chlorine. Indicate their mass numbers and atomic numbers.
Answer:
Numerical Problems (Solved):
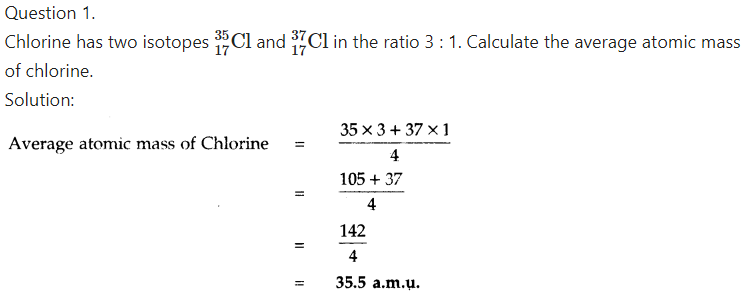
Question 2.
Out of 18X and 16Y which atom is chemically more reactive?
Solution:

No. of valence electrons in X =8
No. of valence electrons in Y =6
∴ Y is more reactive.
Question 3.
An element has 12 neutrons and mass number 23. Give the atomic number and symbol of the element.
Solution:
Solution:
- Atomic number = 9
- Mass number = 19
- No. of protons = 9
- No. of neutrons = 19 – 9 = 10
- Electronic configuration = 2 7
- Valency = 8 – 7 = 1.
Very Short Answer Type Questions
Question 1.
Who discovered canal rays?
Answer:
Goldstein discovered canal rays in 1886.
Question 2.
Which charge is present on canal ray particles?
Answer:
They carry positive charge.
Question 3.
Which charge is present on protons?
Answer:
Positive charge.
Question 4.
Where are protons present in an atom?
Answer:
Protons are present in the nucleus, deep inside the atom.
Question 5.
What were the characteristics of an atom according to Dalton?
Answer:
According to Dalton an atom was indivisible and indestructible.
Question 6.
Which discovery is against Dalton’s theory?
Answer:
Discovering of electrons and protons in an atom.
Question 7.
Who proposed the first model for the structure of atom?
Answer:
J.J. Thomson.
Question 8.
Why did Rutherford used gold foil in a-ray scattering experiment?
Answer:
This is because he wanted as thin a layer as possible. It was about 1000 atoms thick.
Question 9.
What are α-particles?
Answer:
They are doubly charged helium ions (He2+).
Question 10.
What is the charge on α-particles?
Answer:
+2 units.
Question 11.
What is the mass of an α-particle?
Answer:
4 u.
Question 12.
How the defects of Rutherford’s model were removed by Neil Bohr?
Answer:
The electrons revolve around the nucleus in an atom in fixed circular paths called orbits or energy levels and don’t lose energy.
Question 13.
What are energy levels or shells?
Answer:
The definite orbits in where electrons revolve around the nucleus are called energy levels. These are designated as K, L, M, N ……………. 1, 2, 3, 4.
Question 14.
Define mass number of an element.
Answer:
It is the sum of number of neutrons and protons present in the nucleus of the atom of the element.
Question 15.
Who gave the distribution of electrons in the various shells of an atom?
Answer:
Bohr and Bury.
Question 16.
What is the maximum number of electrons present in an electronic shell?
Answer:
2n2 (Where n is the number of shells).
Question 17.
What is the maximum first four shells of an atom?
Answer:
2, 8, 18, 32.
Question 18.
What is the maximum number of electrons in the outermost shell of an atom?
Answer:
8
Question 19.
Which elements are chemically inert?
Answer:
These are the elements which have 8 electrons in their outermost shells.
Question 20.
Define octet.
Answer:
The maximum number of electrons present in the outermost shell of an atom is called octet.
Question 21.
Define valency of an element.
Answer:
It is the combining capacity of an atom of the element and it is equal to the number of electrons lost or gained or contributed for sharing from its valence shell in order to complete its octet.
Question 22.
Define atomic number.
Answer:
It-is the number of protons present in the nucleus of an atom of the element.
Question 23.
Define nucleon.
Answer:
The neutrons and protons present in the nucleus of an atom of the element are collectively known as nucleons.
Question 24.
Where is most of the mass of the element present?
Answer:
In the nucleus of an atom.
Question 25.
Define atomic mass of an element.
Answer:
It is the sum of the number of neutrons and protons present in the nucleus of its atom.
Question 26.
What are isotopes?
Answer:
Atoms of the same element having same atomic number but different mass numbers are called isotopes.
Question 30.
What is the atomic mass of chlorine?
Answer:
35.5
Question 31.
Isotope of which element is used for the treatment of goitre?
Answer:
An isotope of iodine.
Question 32.
Isotope of which element is used in atomic reactor?
Answer:
Uranium.
Question 33.
What are isobars?
Answer:
Atoms of the different elements having same mass number but different atomic numbers are called isobars.

Question 36.
What is the total number of electrons in an atom if its K-and L-shells are fully filled?
Answer:
10 (K-2, L-8).
Question 37.
What electrons in an atom influence its chemical properties?
Answer:
Valence electrons (electrons in the outermost shell).
Question 38.
How will you represent chlorine atom having mass number 35 and atomic number 17?
Answer:
![]()
Question 39.
Name the sub-atomic particle on which size of an atom depends?
Answer:
Electron.
Question 40.
Name the atoms having same atomic number but different mass numbers.
Answer:
Isotopes.
Question 41.
Mg2+ has 10 electrons, what is the number of protons in it?
Answer:
10 + 2 = 12.
Question 42.
Name the element which has no neutrons in the nucleus of its atom.
Answer:
Hydrogen.
Question 43.
Out of electrons, protons and neutrons, which are same in isotopes?
Answer:
Electrons and protons.

Question 45.
An element has atomic number 16. Give its electronic configuration and number of valence electrons.
Answer:
Electronic configuration 2, 8, 6.
Question 46.
The number of electrons in the valence shell of sodium (Na) is ……………… .
Answer:
1.
Question 47.
Fluorine belongs to …………… family.
Answer:
Halogen.
Question 48.
In an atom, the number of …………… is the same as the number of ………………. .
Answer:
Electrons, protons
Question 49.
Sodium has …………….. electron …………….. than sodium ion.
Answer:
One, more
Question 50.
Isotopes of an element are …………….. because they have ……….. number of electrons.
Answer:
Chemically similar, same
Question 51.
The number of electrons in the valence shell of an atom cannot be ………………..>
Answer:
More than eight.
Science Guide for Class 9 PSEB Structure of the Atom InText Questions and Answers
Question 1.
What are canal rays?
Answer:
A beam of rays or stream of particles which travel in a direction away from anode, towards cathode, when any gas taken in a discharge tube is subjected to the action of high voltage under low pressure are called canal rays.
Question 2.
If an atom contains one electron and one proton, will it carry any charge or not?
Answer:
No, it will not carry any charge.
Question 3.
On the basis of Thomson’s model of an atom explain how the atom is neutral as a whole.
Answer:
Thomson’s model of atom.
1. According to Thomson, an atom may be regarded as a uniform sphere of positive electricity (protons) in which negatively charged electrons are embedded like the seeds in a watermelon.
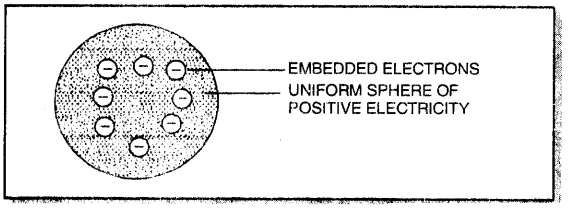
2. The total positive charge is equal to the total negative charge on all the electrons so that atoms on the whole is electrically neutral.
Question 4.
On the basis of Rutherford’s model of an atom which sub-atomic particle is present in the molecule of an atom?
Answer:
Proton.
Question 5.
Draw a sketch of Bohr’s model of an atom with three shells.
Answer:
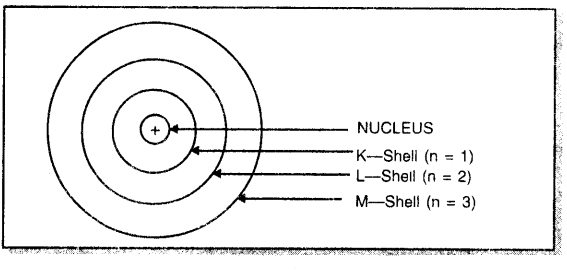
Question 6.
What do you think would be the observation if the ∝-particle scattering experiment is carried out using a foil of metal other than gold?
Answer:
Almost all the ∝-particles will pass undeflected and hardly any a-particle is deflected.
Question 7.
Name the three sub-atomic particles of an atom.
Answer:
These are:
(a) Electron
(b) Proton and
(c) Neutron
Question 8.
Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Answer:
Atomic mass of Helium = 4 u No. of protons = 2
∴ No. of neutrons = 4 – 2 = 2
Question 9.
Write the distribution of electrons in carbon and sodium atoms.
Answer:
| Element | At. No. | Electronic Configuration | ||
| K | L | M | ||
| Carbon | 6 | 2 | 4 | 1 |
| Sodium | 11 | 2 | 8 | 1 |
Question 10.
If K and L shell of an atom are full then what would be the total number of electrons in it?
Answer:
- No. of electrons in K-shell = 2
- No. of electrons in L-shell = 8
- Total no. of electrons = 2 + 8 = 10
Question 11.
How will you find the valency of chlorine, sulphur and magnesium?
Answer:
Chlorine has the electronic configuration = 2, 8, 7
∴ Valency of chlorine = 8 – 7 = 1
Sulphur has the electronic configuration = 2, 8, 6
∴ Valency of sulphur = 8 – 6 = 2
Magnesium has the electronic configuration = 2, 8 ,2
∴ Valency of magnesium = 2
Question 12.
If number of electrons in an atom are 8 and number of protons are also 8, then ;
1. What is the atomic number of the atom?
2. What is the charge on the atom?
Answer:
1. No. of protons = 8
∴ Atomic no. of the element = 8
2. Zero.
Question 13.
With the help of table 4.1 (given in Text Book) find out the mass number of oxygen and sulphur atom.
Answer:
- Mass number of oxygen = 8 + 8 = 16
- Mass number of sulphur = 16 + 16 = 32
Question 14.
For the symbols H, D and T tabulate three fundamental particles found in each of them.
Answer:
| Symbol of element | Atomic number | Mass No. | No. of electrons | No. of protons | No.of Neutrons |
| H | 1 | 1 | 1 | 1 | 0 |
| D | 1 | 2 | 1 | 1 | 1 |
| T | 1 | 3 | 1 | 1 | 2 |
Question 15.
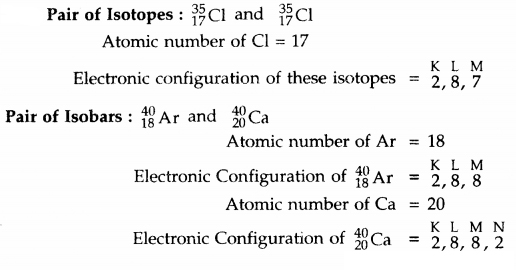
Write the electronic configuration of any one pair of isotopes and isobars.
Answer:
PSEB 9th Class Science Guide Structure of the Atom Textbook Questions and Answers
Question 1.
Compare the properties of electron, proton and neutron.
Answer:
| Particle | Charge (e) | Mass (m) | Charge/Mass (e/m) |
| Electron | – 1.6022 × 1019C | 9.109 × 1028g | – 1.76 × 108 |
| Proton | + 1.6022 × 10-19C | 1.672 × 10-24g | + 9.58 × 104 |
| Neutron | Zero | 1.675 × 10-24g | Zero |
Question 2.
What are the limitations of J.J. Thomson model of atom?
Answer:
Limitations of J.J. Thomson model of atom
- It could not explain stability of the atom.
- It could not explain hydrogen spectrum.
Question 3.
What are the limitations of Rutherford’s model of atom?
Answer:
Limitations of Rutherford’s model of atom
- It can’t explain stability of the atom.
- It can’t explain hydrogen spectrum.
Question 4.
Describe Bohr’s model of atom.
Answer:
The main points of Bohr’s model of atom are:
1. An atom has three types of particles called fundamental particles. These are electrons, protons and neutrons.
2. The protons and neutrons are present in the nucleus, present in the centre. The electrons are present around the nucleus and at very large distances from it. There is a large vacant space between the nucleus and the electrons.
3. An atom is electrically neutral as the number of protons is equal to the number of electrons.
4. The electrons are revolving around the nucleus in fixed circular paths which are called energy levels or energy shells or orbits. The energy levels or energy shells can be counted in two ways 1, 2, 3, 4, 5, 6 or as K, L, M, N, O, P. The counting starts from the centre outwards.
5. The various energy levels are arranged in order of increasing energy. The order of energy is 1 < 2 < 3 < 4 < 5 or K < L < M < N < O ………..
6. The energy of an electron in an atom is quantised.
7. There is no change in the energy of the electrons as long as they keep on revolving in the same energy level.
8. The angular momentum of an electron in an atom is quantised.
9. The change in energy can take place only when the electron jumps from one energy level to the other. If the electron gains energy from outside, it jumps from lower energy to the higher energy level. If the electron jumps from higher energy level to the lower energy level, it loses energy.
Question 5.
Compare all the proposed models of atom given in the chapter 3 of the text.
Answer:
Comparing Thomson’s model, Rutherford’s model and Bohr’s model of atom.
A. Thomson’s model of atom.
1. According to Thomson, an atom may be regarded, a uniform sphere of positive electricity (protons) in which negatively charged electrons are embedded like the seeds in a watermelon.
2. The total positive charge is equal to the total negative charge on all the electrons so that atoms on the whole is electrically neutral.
B. Rutherford’s nuclear model of atom.
The main features of Rutherford’s nuclear model of atom are:
- Atom has a dense, heavy, positively charged central part called nucleus.
- The electrons are present at very large distances from the nucleus.
- The total positive charge on the nucleus is equal to the total ~ve charge on all the electrons so that atom on the whole is electrically neutral.
- The electrons are revolving around the nucleus so that attractive force of the nucleus is balanced by the centrifugal force (just like planets revolve around the sun).
- It is different from Thomson’s model, because in Thomson’s model of atom, the total mass, positive charge and electrons are uniformly distributed
C. Bohr’s model of atom. Described in given above.
Question 6.
Summarize the rules for writing of distribution of electrons in various shells for the first eighteen elements.
Answer:
The various rules for writing the electronic configuration of first elements are:
1. The energy levels or energy shells are filled in order of increasing energies. The electrons first enter the K shell (n =
- which is closest to the nucleus. This is followed by L shell (n = 2), M shell (n = 3) and so on. Here V represents the number of the shell.
- The maximum number of electrons in any shell is given as 2n2 (n = No. of shells).
- The outermost energy shell cannot have more than 8 electrons. The next inner shell called penultimate shell cannot have more than 18 electrons.
- It is not necessary that an energy level or shell is fully filled before the filling in the next energy level starts. In fact filling of electrons in a new shell starts when any shell contains 8 electrons.
Question 7.
Define valency by taking examples of silicon and oxygen.
Answer:
Valency: It is the combining capacity of an atom of the element and is numerically equal to the number of electrons present in the valence shell of an atom (if the no. of electrons is 1 to 4) or eight minus the number of electrons present in the valence shell of an atom (if the no. of electrons is more than 4).
e.g. Silicon (14Si) has electronic configuration = 2, 8, 4
∴ its valency = 4 (No. of electrons in its valence shell)
Oxygen (8O) has electronic configuration = 2, 6
∴ its valency = 8 – 6 = 2 (8 – No. of electrons in the valence shell)
Question 8.
Explain with examples
- Atomic number
- Mass number
- Isotopes
- Isobars. Give any two uses of isotopes.
Answer:
- Atomic number. It is the number of protons present in the nucleus of an atom of an element.
- Mass number. It is the sum of total number of protons and neutrons present in the nucleus of an atom.
- Isotopes. These are the atoms of the same element having same atomic number but different mass numbers.
- Isobars. These are the atoms of different elements having same mass number but different atomic numbers.

Question 9.
Na+ has completely filled K and L shells. Explain.
Answer:
Na has atomic number =11
∴ Na atom has electrons =11
Na+ ion has electrons = 11 – 1 = 10
The electronic configuration of Na+ = 2, 8
∴ In Na+, K and L-shells are completely filled.
Question 10.
If bromine atom is in the form of say two isotopes 35 Br (49.7%) and 35 Br (50.3%), then calculate the average mass of bromine atom.
Answer:
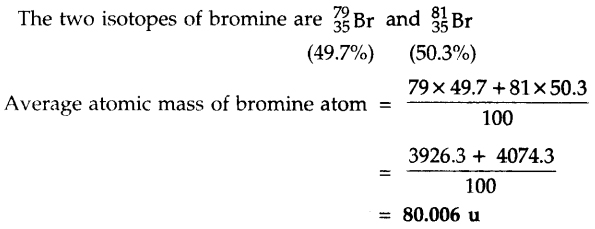
Question 11.
The average atomic mass of a sample of an element X is 16.2 u, what are the percentages of isotopes g X and “X in the sample?
Answer:
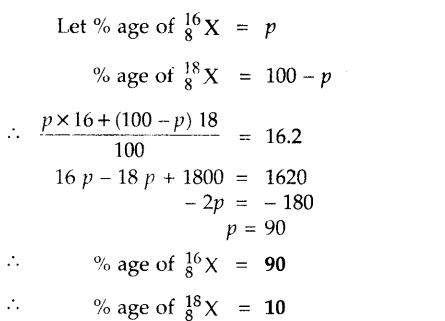
Question 12.
If Z = 3, what would be the valency of the element? Also, name the element.
Answer:
Z = 3
- Atomic no. of element = 3
- No. of electrons in one atom of the element = 3
- Atomic no. of the element (Z) = 3
- Its electronic configuration = 2, 1
- Hence its valency = 1 (No. of electrons in the valence shell.)
- The name of the element = Lithium.
Question 13.
Composition of the nuclei of two atomic species X and Y are given as under:

Give the mass numbers of X and Y. What is the relation between the two species?
Answer:
- Mass no. of X = 6 + 6 = 12
- Mass no. of Y = 6 + 8 = 14
- The two species are isotopes because they have same atomic number but different mass numbers.
Question 14.
For the following statements write T for True and F for False.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about 1/2000 times that of proton.
(d) Isotope of iodine is used for making tincture iodine, which is used as a medicine.
Answer:
(a) F
(b) F
(c) T
(d) F.
Put tick (✓) against correct choice and cross (×) against wrong choice in questions 15, 16 and 17
Question 15.
Rutherford’s alpha-particle scattering experiment was responsible for the discovery of :
(a) Atomic Nucleus
(b) Electron
(c) Proton
(d) Neutron
Answer:
(a) Atomic Nucleus (✓)
(b) Electron (×)
(c) Proton (×)
(d) Neutron (×)
Question 16.
Isotopes of an element have:
(a) the same physical properties
(b) different chemical properties
(c) different number of neutrons
(d) different atomic numbers.
Answer:
(a) the same phvsicul properties (×)
(b) different chemical properties (×)
(c) different number of neutrons (✓)
(d) different atomic numbers (×)
Question 17.
Number of valence electrons in Cl+ ion are:
(a) 16
(b) 8
(c) 17
(d) 18
Answer:
(a) 16 (×)
(b) 8 (×)
(c) 17 (×)
(d) 18 (×).
Question 18.
Which one of the following is a correct electronic configuration of sodium?
(a) 2, 8
(b) 8, 2, 1
(c) 2, 1, 8
(d) 2, 8, 1.
Answer:
(d) 2, 8, 1.
Question 19.
Complete the following table:
| Atomic Number | Mass Number | Number of Neutrons | Number of Protons | Number of Electrons | Name of the Atomic Species |
| 9 | – | 10 | ~ | – | – |
| 16 | 32 | – | – | – | Sulphur |
| – | 24 | – | 12 | – | – |
| – | 2 | – | 1 | – | – |
| – | 1 | 0 | 1 | 0 | – |
Answer:
| Atomic Number | Mass Number | Number of Neutrons | Number of protons | Number of Electrons | Name of the Atomic Species |
| 9 | 19 | 10 | 9 | 9 | Fluorine |
| 16 | 32 | 16 | 16 | 16 | Sulphur |
| 12 | 24 | 12 | 12 | 12 | Magnesium |
| 1 | 2 | 1 | 1 | 1 | Deuterium |
| 1 | 1 | 0 | 1 | 0 | Protium |
Follow on Facebook page – Click Here
Google News join in – Click Here
Read More Asia News – Click Here
Read More Sports News – Click Here
Read More Crypto News – Click Here