WBBSE 10th Class Science Solutions Physical Science & Environment Chapter 3 Chemical Calculations
WBBSE 10th Class Science Solutions Physical Science & Environment Chapter 3 Chemical Calculations
West Bengal Board 10th Class Science Solutions Physical Science & Environment Chapter 3 Chemical Calculations
WBBSE 10th Class Physical Science & Environment Solutions
Synopsis
- The chemical substances participating in a chemical reaction are known as reactants and the substances produced in the reaction are called products.
- Chemical equation is the representation of a chemical reaction with the help of symbols and formulas. The reactants are written on the left side while the products are written on the right side of the equation.
- A chemical equation gives us qualitative as well as quantitative information about the reaction. We can calculate mass, number of moles and volumes of the involved reactants and products from the chemical equation.
- Law of conservation of mass: According to the law of conservation of mass proposed by Lavoisier, in any physical or chemical change total mass of all the substances before the change is equal to the total mass of all the substances formed after the change.
- Law of conservation of mass and energy: The law states that the total amount of mass and energy remains constant before and after any chemical or physical transformation.
- Equivalence of mass and energy: According to Einstein’s theory of relativity, mass and energy are different aspects of the same entity and are interconvertible. If a substance ‘A’ of mass ‘m’ is converted into another substance ‘B’ to produce an equivalent amount of energy ‘E’, then according the Einstein’s equation of mass-energy equivalence, E = mc², where c = velocity of light in vacuum.
- In normal chemical reactions very minute change of mass takes place, which is too small to be measured. Hence, the law of conservation of mass and energy is not applicable for a general chemical reaction. However, the law is applicable for nuclear reactions where change in energy is detectable.
- The mass (measured in gram unit) of 1 L of a gas at STP is known as standard density of the gas.
- The vapour density or relative density of a gas is defined as the ratio of mass of given volume of the gas at a given temperature and pressure to the mass of equal volume of hydrogen gas at the same temperature and pressure.
- Vapour density is a ratio and hence, it has no unit.
- Vapour density is independent of temperature and pressure. So, its value is constant at any temperature or pressure.
- Molecular mass of a gas is twice its vapour density.
- At STP, the volume of 1 mol of any gas is 22.4 L.
- At STP, the mass of 1 L of any gas vapour density of the gas × 0.089
- Calculation of relative quantities of reactants and products in a chemical reaction is known as stoichiometry.
SHORT AND LONG ANSWER TYPE QUESTIONS
Q.1 Discuss the significance of a balanced chemical equation in chemical calculation.
Ans. A chemical equation provides us with qualitative as well as quantitative information about a chemical reaction.
Qualitative information: From the chemical equation we can easily identify the reactants and products in a chemical reaction.
Quantitative information:
- It gives us an idea about the number of atoms and molecules of reactants and products involved in the reaction.
- It gives us an idea about the mass of the reactants that reacts with each other and the mass of the products formed.
- If the reactants or products are gases, then at a given temperature and pressure, their volume can be determined.
Q.2 What information can be obtained from the equation 2H2 + O2 → 2H2O ?
Ans. The equation provides us with the following information-
Qualitative information: Hydrogen and oxygen react with each other to form water.
Quantitative information:
- 2 mol of hydrogen reacts with 1 mol of oxygen to produce 2 mol of water.
- Here, 2 × (2 × 1)g = 4 g hydrogen reacts with (2 × 16)g = 32g oxygen to produce 2 × (2 × 1 + 16) = 36g water.
- At the same temperature and pressure, 2 volumes of hydrogen combine with 1 volume of oxygen to produce 2 volumes of water vapour.
- At STP, 2 × 22.4L = 44.8L hydrogen combines with 22.4L oxygen to produce 2 × 22.4 L = 44.8L steam.
Q.3 State the limitations of a chemical equation.
Ans. A chemical equation cannot give us information about-
- Concentration of reactants and products.
- The rate of the reaction or the time required for completion of the reaction.
- Whether the reaction has completed or not.
Q.4 In chemical reaction mass is conservede-xplain.
Ans. In a chemical reaction, reactants are transformed into products. Total mass of the products is found to be equal to the total mass of the reactants.
Let us consider, A and B reacts with each other to produce C and D in a reaction. Hence,
mass of A+ mass of B = mass of C + mass of D Thus, total mass of A and B total mass of C and D.
Q.5 Show with the help of an experiment that mass is conserved in a chemical reaction.
Ans. The law of conservation of mass can be proved with the help of rusting of iron.
Materials required: A hard glass test tube, some new iron nails, water, rubber cork, common balance.
Experiment: A small amount of water is taken in a hard glass test tube and some glossy iron nails are dipped into the water. The mouth of the test tube is covered with the help of rubber cork. The mass of the test tube is measured with a common balance and is left undisturbed for some days.
Observation: After some days it will be observed that brown coloured rust is formed on the iron nails. In this condition, the mass of the test tube is measured again with a common balance and it is observed that the mass of the test tube before and after the experiment is equal.
Conclusion: Iron nails react with oxygen and water present in the test tube to form rust.
Fe + O2 + water vapour → Fe2O3 · xH₂O,
x = number of water molecules.
In this case, total mass of the iron piece along with unreacted oxygen and water vapour is equal to the mass of the rusted iron. Thus, we can say that mass is conserved during chemical reactions.
Q.6 A piece of iron gets heavier when it is kept in moist air for a long time- state whether mass is conserved in this case?
Ans. When iron is kept in moist air for a long time rust is formed over the surface of the iron piece. Oxygen and water vapour present in air reacts with iron to form hydrated ferric oxide (Fe2O3 · xH₂O). As a result, the mass increases. So, total mass of the iron piece along with oxygen and water vapour is equal to the mass of rusted iron. Hence, mass is conserved in this process.
Q.7 On burning, mass of candle decreases-state whether mass is conserved ass is c in this process.
Ans. On burning, the wax of the candle reacts with aerial oxygen to form carbon dioxide (CO2), other gases and water vapour. These products escape into atmosphere. If all the products are collected together, then it would be observed that the total mass of the products is equal to the total mass of wax and used oxygen. Thus, it can be said mass of wax + mass of used oxygen before burning = mass of remaining candle + mass of produced CO2 + mass of other gases produced + mass of water vapour.
Hence, mass is conserved in this process.
Q.8 Explain with reasons whether law of conservation of mass is followed during electrolysis of acidified water.
Ans. During electrolysis of acidified water, water decomposes to form hydrogen and oxygen. If electrolysis is carried out in a closed vessel it will be observed that the total mass remains same before and after the electrolysis, i.e., total mass of acidified water = mass of remaining acidified water + mass of produced hydrogen + mass of produced oxygen. So, it can be said that law of conservation of mass is obeyed during electrolysis of acidified water.
Q.9 In the normal chemical reactions mass does not undergo measurable change-explain.
Ans. In the normal chemical reactions, heat is either evolved or absorbed. According to mass energy equivalence, if heat is evolved in a reaction, equivalent amount of mass of the reactant will decrease. On the other hand, if heat is absorbed, equivalent amount of mass of the reactant will increase. But, in normal chemical reactions, the change in mass observed due to the absorption or evolution of heat is too small to be measured with a balance. Hence, we can say that no measurable change of mass takes place in a chemical reaction.
Q.10 What is the significance of the equation E = mc2 ? In normal chemical reactions heat is either evolved or absorbed, yet, the change of mass is not observed. Why?
Ans. When a substance of mass ‘m’ is converted into another substance, an equivalent amount of energy E is produced. The equation, E = mc², represents the conversion of mass into energy and vice versa, where c = velocity of light in vacuum.
The change of mass involved in the normal chemical reactions is too small to be measured. Thus, change of mass is not generally observed in normal chemical reactions.
Q.11 Law of conservation of mass is not applicable in high energy transformations-explain.
Ans. During high energy transformations such as in nuclear reactions, the total mass is converted into an equivalent amount of energy. As a result, the large amount of energy produced can be determined from Einstein’s equation, E = mc². Hence, it can be said that in high energy transformations law of conservation of mass is not valid. Instead, in such cases, law of conservation of mass and energy is applicable.
Q.12 1g of a substance is completely converted into energy. Using the equation E = mc² find the amount of energy obtained due to the conversion.
Ans. The equation, E = mc2, represents massenergy equivalence where,
E = energy, m = mass and c = speed of light in vacuum = 3 × 1010 cm · s-1

Q.13 What do you mean by standard density of a gas? What is vapour density or relative density of a gas?
Ans. The mass (measured in gram unit) of 1 litre of a gas at STP is known as standard density of the gas.
The vapour density or relative density of a gas is defined as the ratio of mass of given volume of the gas at a given temperature and pressure to the mass of equal volume of hydrogen gas at the same temperature and pressure.
Q.14 Deduce the relationship between standard density and vapour density of a gas.
Ans. Vapour density of a gas
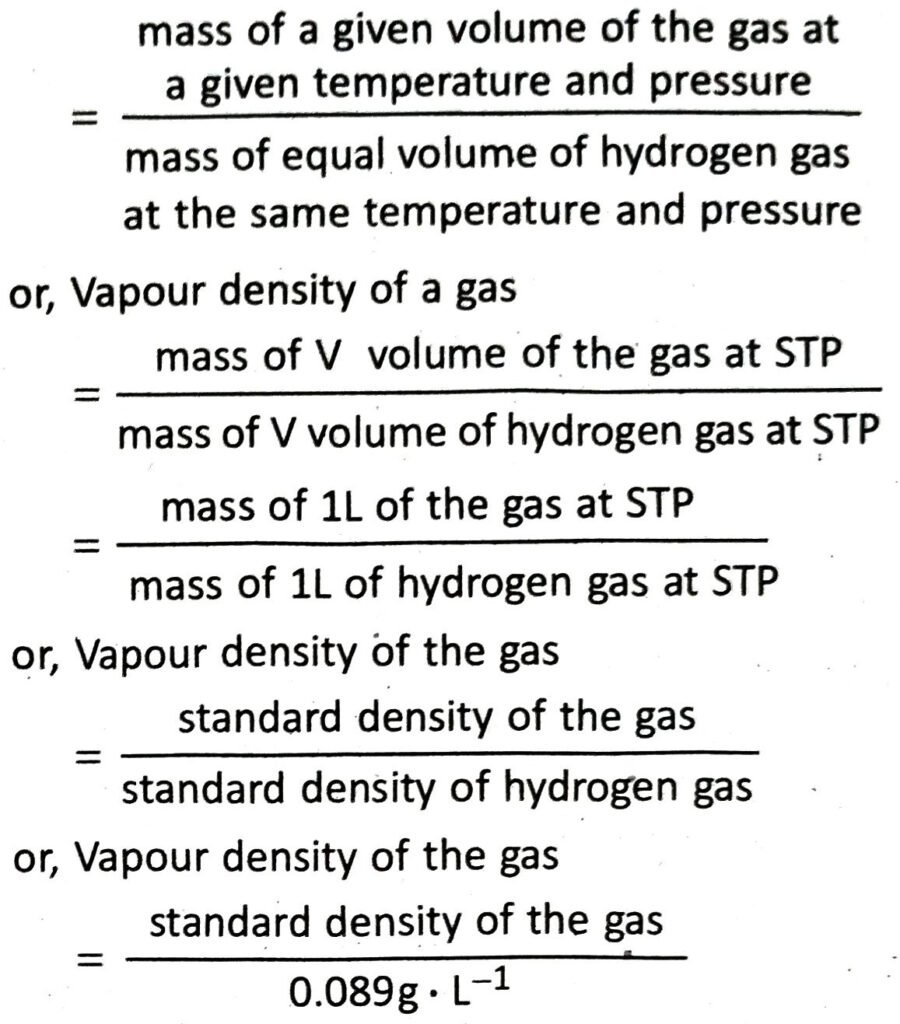
Q.15 How will you understand whether a gas is heavier than air or not? Vapour density of chlorine is 35.5. How will it displace air from a gas jar?
Ans. If the vapour density of a gas is found to be greater than that of air (14.4), it can be assumed that the gas is heavier than air.
The vapour density of chlorine is greater than air (14.4). Hence, it is heavier than air. So, there will be upward displacement of air when chlorine gas is collected into a gas jar filled with air.
Q.16 Vapour density of a gas does not change with temperature-justify.
Ans. The vapour density or relative density of a gas is defined as the ratio of mass of given volume of the gas at a given temperature and pressure to the mass of equal volume of hydrogen gas at the same temperature and pressure. With increase or decrease in temperature the volumes of hydrogen as well as the concerned gas increases or decreases in the same proportion. Hence, vapour density of a gas doesnot change with temperature.
Q.19 Dry air is heavier than moist air. Explain of than moist with reason.
Ans. The average vapour density of air is 14.4 while that of water vapour is 9. Hence, a given volume of dry air is heavier than equal volume of water vapour. Moist air contains more amount of water vapour as compared to dry air. That is why dry air is heavier than moist air.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. Law of conservation of mass was proposed by
A. Dalton
B. Lavoisier
C. Arrhenius
D. Proust
Ans. B
2. Difference in mass between reactants and products is
A. observed in low energy transformations
B. observed in general chemical reactions
C. observed in high energy transformations
D. never observed
Ans. C
3. Two substances, A and B reacts with each other to produce C and D. Which of the following statements is true?
A. total mass of A and B > total mass of C and D
B. total mass of A and B < total mass of C and D
C. total mass of A and B = and D total mass of C
D. mass of A = mass of B and mass of C = mass of D
Ans. C
4. The mass-energy equivalence is represented by the equation
A. E2 = mc
B. E = mc²
C. E = m²c
D. E = mc
Ans. B
5. Which of the following cannot be determined from a chemical equation?
A. number of moles of reactants & products
B. volume of reactants and products at STP
C. concentration of reactants and products
D. mass of reactants and products.
Ans. C
6. The equation E = mc2 is related to
A. Newton
B. Planck
C. Avogadro
D. Einstein
Ans. D
7. Measurable change in mass takes place in
A. general chemical reactions
B. nuclear reactions
C. exothermic reactions
D. endothermic reactions
Ans. B
8. In the equation E = mc2, the terms E, m and c respectively represents
A. mass, energy and speed of light in vacuum
B. speed of light in vacuum, mass and energy
C. energy, mass and speed of light in vacuum
D. energy, speed of light in vacuum and mass
Ans. C
9. According to mass-energy equivalence, in an exothermic reaction the mass of products
A. will decrease in equivalent amount
B. will increase in equivalent amount
C. will increase or decrease in equivalent amount
D. will remain unchanged
Ans. A
10. According to mass-energy equivalence, in an endothermic reaction the mass of products
A. will decrease in equivalent amount
B. will increase in equivalent amount
C. will increase or decrease in equivalent amount
D. will remain unchanged
Ans. B
11. Total amount of mass and energy after any physical or chemical transformation
A. decreases
B. increases
C. remains constant
D. none of these
Ans. C
12. The amount of N2 produced from 64g NH4NO2 is
A. 14 g
B. 28 g
C. 32 g
D. 40 g
Ans. B
13. The volume of NO2 produced when 2 mol of NO reacts with O2 at STP is
A. 11.2 L
B. 22.4 L
C. 44.8 L
D. 5.6 L
Ans. C
14. Which of the following relations is correct?
A. at STP, mass of 1L of a gas = vapour density of the gas × 0.89
B. at STP, mass of 1L of a gas = vapour density of the gas × 0.089
C. vapour density of a gas = 0.089 × mass of 1L of a gas at STP
D. vapour density of a gas = 2.24 × molecular mass of the gas
Ans. B
15. 1 mol nitrogen combines with 3 mol of hydrogen to produce
A. 1 mol NH3
B. 2 mol NH3
C. 3 mol N3
D. 4 mol NH3
Ans. B
16. The relationship between molecular mass and vapour density is given by
A. 2M=D
B. M=D2
C. M = 3D
D. M = 2D
Ans. D
17. The vapour density of SO2 is
A. 16
B. 32
C. 64
D. 48
Ans. B
18. The molecular mass of a gas is 46. Its vapour density is
A. 23 u
B. 23 amu
C. 23 g
D. 23
Ans. D
19. Vapour density of a gas is 22. Volume of 44g of the gas at STP is
A. 11.2 L
B. 5.6 L
C. 22.4 L
D. 44.8 L
Ans. C
20. Average vapour density of air is
A. 14.4
B. 16.5
C. 17.2
D. 20.1
Ans. A
21. The molecular mass of chlorine is 71. Which of the following statements is correct?
A. chlorine gas is lighter than air
B. chlorine gas is heavier than air
C. chlorine gas has almost same mass as that of air
D. unpredictable
Ans. B
22. Mass of 112 ml O2 at NTP is
A. 0.64 g
B. 0.96 g
C. 0.32 g
D.0.16 g
Ans. D
23. Mass of 6.022 x 1022 number of molecules of water is
A. 18 g
B. 1.8 g
C. 9 g
D. 0.18 g
Ans. B
24. Volume of CO2 gas at STP, obtained by heating 1 mol of CaCO3 is
A. 22.4 L
B. 11.2 L
C. 5.6 L
D. 44.8 L
Ans. A
25. The percentage-increase in mass of a Mg wire after its combustion in pure oxygen is
A. 100%
B. 66.7%
C. 24%
D. 80%
Ans. B
26. The volume of hydrogen at STP obtained from the reaction of 4 mol Na with water is
A. 22.4 L
B. 44.8 L
C. 11.2L
D. 5.6 L
Ans. B
27. Number of moles of NH3 gas formed by the reaction of 2 mol N2 with 6 mol H2 is
A. 6 mol
B. 2 mol
C. 8 mol
D. 4 mol
Ans. D
28. Vapour density of sulphur trioxide is
A. 40
B. 32
C. 36
D. 80
Ans. A
29. Vapour density of a gas is 32. Which of the following is the molecular mass of the gas?
A. 8
B. 16
C. 32
D. 64
Ans. D
30 The volume of CO2 at STP, obtained by the complete combustion of 1.2 g of C is
A. 1.2 L
B. 2.24 L
C. 4.8 L
D. 4.4 L
Ans. B
31. Molecular mass of methane is 16. Which of the following is its vapour density?
A. 22.4
B. 8
C. 16
D. 32
Ans. B
Answer in brief
1. What do you mean by ‘conservation of mass’ in a chemical reaction?
Ans. In a chemical reaction, total mass of all the reactants is equal to total mass of all the products. This is known as ‘conservation of mass’ in a chemical reaction.
2. State the law of conservation of mass and energy.
Ans. The law of conservation of mass and energy states that the total mass and energy before and after a transformation is always constant.
3. When is the law of conservation of mass and energy’ applicable instead of ‘law of conservation of mass’?
Ans. In high energy transformations like nuclear reactions, ‘law of conservation of mass and energy’ is applied instead of law of conservation of mass’.
4. Define ‘reactants’ and ‘products’.
Ans. The chemical substances participating in a chemical reaction are known as ‘reactants’. The substances produced due to chemical reaction among the reactants are known as ‘products’.
5. Is the change in mass detectable in normal chemical reactions?
Ans. No, the change in mass is not detectable in normal chemical reactions.
6. Write the relationship between molecular mass (M) and vapour density (D) of a gas.
Ans. Molecular mass (M) = 2 × vapour density (D).
7. Vapour density of SO2 gas at 25°C and normal pressure is 32. What will be its vapour density at 50°C?
Ans. Since, vapour density is independent of temperature and pressure, hence, the vapour density of SO₂ at 50°C will remain 32.
8. Define ‘stoichiometry’.
Ans. Stoichiometry is defined as the calculation of relatve quantities of reactants and products in a chemical reaction.
9. What is the unit of vapour density.
Ans. Since vapour density is a ratio, thus it has no unit.
10. Mass of 5.6 L of a gas at STP is 11g. Find the vapour density of the gas.
Ans. Mass of 5.6 × 4 = 22.4 L of the gas at STP is 11 × 4 = 44g. Hence, vapour density of the gas= 44/2 = 22.
11. Name the physical quantity whose unit is ‘mole’.
Ans. ‘Mole’ is the SI unit of the amount of substance.
12. What is the number of molecules in 22.4 L of a gas at STP?
Ans. 6.022 × 1023.
13. What is the number of moles in m g of a gas having molar mass of M g/mol.
Ans. Number of moles of the said gas = m/M.
14. What is the number of molecules in 1.8 g water?
Ans. Number of molecules present in 1.8 g water
= 6.022 × 1023 × 1.8/18 = 6.022 × 1022.
15. How much ammonia is equivalent to 4 mol ammonia ?
Ans. 4 mol ammonia is equivalent to 17 × 4 = 68 g of ammonia.
16. What is the relationship between standard density and vapour density?
Ans. Standard density = 0.089 × vapour density.
Fill in the blanks
1. A chemical equation provides us with ………… and ………. information.
Ans. quantitative, qualitative
2. Velocity of light in vacuum is …………
Ans. 3 × 108m · s-1
3. The change in mass is ………….. in normal chemical reactions.
Ans. undetectable
4. Mass is …………. in case of rusting of iron.
Ans. conserved
5. The volume of H2S produced from 88g FeS is ………….
Ans. 22.4 L
6. When hydrogen reasts with oxygen to form water, the number of moles …………
Ans. decreases
7. Vapour density is …………….. of temperature and pressure.
Ans. independent
8. CO2 is heavier than air as the ……………… of CO2 is greater than air.
Ans. vapour density
9. If the vapour density of a gas is 20, the number of moles of 80g of the gas is …………
Ans. 2
10. The mass of 1L H2 at STP is ……………
Ans. 0.089 g
11. In a chemical reaction, total mass of ………… = total mass of the ………..
Ans. reactants, products
12. The proposer of E=mc2 equation is ………………
Ans. Albert Einstein
13. At STP, 22.4 L of H2 reacts with 22.4 L of Cl2 to produce …………. L of HCl.
Ans. 44.8
14. Vapour density is a …………. less quantity.
Ans. unit
State whether true or false
1. The total mass remains constant before and after a chemical reaction.
Ans. True
2. The vapour density of a gas is twice its molecular mass.
Ans. False
3. 35Cl atom is taken as the standard for the determination of atomic mass.
Ans. False
4. 8g oxygen contains 3.011 × 1023
Ans. False
5. Vapour density is a unitless quantity.
Ans. True
6. 16g oxygen is equal to 0.5 mole oxygen.
Ans. True
7. 6.022 × 1023 molcules of water weighs 18g.
Ans. True
8. The substances produced in a chemical reaction are called reactants.
Ans. False
9. The volume of 1g gas at STP is 22.4L.
Ans. True
10. Scientist Lavoisier proved the law of conservation of mass’.
Ans. True
11. Interconversion of mass and energy is possible.
Ans. True
12. At STP, the mass of 22.4 L of any substance refers to its molecular mass.
Ans. False
13. The amount of O₂ formed by heating 4 mol of KClO3 is 2 mol.
Ans. False
14. Vapour density of a gas × 0.089 = standard density of the gas
Ans. True
15. If the molecular mass of a gas is 44, its vapour density will be 24.
Ans. False
Follow on Facebook page – Click Here
Google News join in – Click Here
Read More Asia News – Click Here
Read More Sports News – Click Here
Read More Crypto News – Click Here