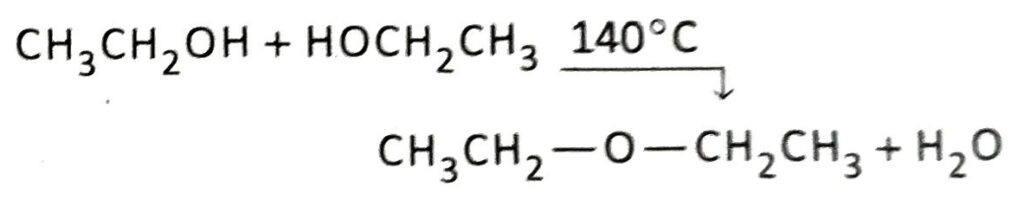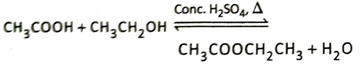WBBSE 10th Class Science Solutions Physical Science & Environment Chapter 8.6 Organic Chemistry
West Bengal Board 10th Class Science Solutions Physical Science & Environment Chapter 8.6 Organic Chemistry
WBBSE 10th Class Physical Science & Environment Solutions
8.6 Organic Chemistry
Synopsis
General Properties of Organic Compounds
- In 1828, a German chemist Friedrich Wohler synthetically prepared urea, an organic compound found in the urine of most mammals, by heating an inorganic compound, ammonium cyanate. This accidental synthesis gave a fatal blow to the ‘vital force theory’ in which it was considered impossible to prepare an organic compound in the laboratory from inorganic compounds.
- The fundamental element of all organic compounds is carbon. Apart from carbon, H, O, N, S, P, halogens and some metals may also be present in organic compounds.
- The self-linking property of carbon by virtue of which its atoms mutually combine with each other to form long open chains (straight or branched) and rings is called catenation. Using its catenation property, carbon forms a vast number of organic compounds.
- Organic compounds in which all the adjacent carbon atoms are linked to one another by single bonds only are called saturated compounds. For example, methane, ethane.
- Organic compounds which contain at least one carbon-carbon double bond or triple bond are called unsaturated compounds. For example, ethylene, acetylene.
- Organic compounds containing carbon and hydrogen are called hydrocarbons.
- An atom or a group of atoms present in an organic compound, which determines the nature and characteristic chemical properties of the compound is called a functional group.
- The phenomenon of existence of two or more compounds possessing the same molecular formula but different physical and chemical properties is called isomerism. Such compounds showing isomerism are called isomers.
- The compounds having the same molecular formula but different structures are called structural isomers and the phenomenon isc known as structural isomerism.
- A scientific and systematic approach of naming the organic compounds was adopted in a conference attended by chemists from worldwide. These rules of naming organic compounds are collectively known as IUPAC (International Union of Pure and Applied Chemistry) nomenclature of organic compounds. These rules have been modified from time to time.
TOPIC – A
General Properties of Organic Compounds
SHORT AND LONG ANSWER TYPE QUESTIONS
Q.1 Write three differences between organic and inorganic compounds.
Ans. The major differences between organic and inorganic compounds are as follows—
| Property |
Organic compound |
Inorganic compound |
| 1. Constituents |
All organic compounds must contain carbon. Apart from carbon, elements like H, N, O, S, P, halogens etc., may also be present in organic compounds. Existence of more than 20 lakhs of organic compounds is known till date. |
Inorganic compounds may or may not contain carbon. About 92 naturally occurring elements and some synthetically prepared elements form almost 90000 inorganic compounds which is far less than the number of organic compounds. |
| 2. Melting point and boiling point |
Organic compounds being covalent in nature generally have low melting and boiling points. |
Most inorganic compounds being ionic in nature have high melting and boiling points. |
| 3. Solubility |
Organic compounds are soluble in organic and non-polar solvents like benzene, CCl4 etc., but insoluble in polar solvents such as water. |
Inorganic compounds are generally soluble in polar solvents such as water but insoluble in non-polar solvents such as benzene. |
Q.2 The general molecular formula of some Isomers is C4Hg. Predict the structural formula of those compounds.
Ans. The general molecular formula of the isomers is C4H8. The formula conforms to the general formula CnH₂n (where n = 4) which belongs to the alkene family. Therefore, the possible isomers and their structural formulas are as follows:
Q.3 What is homologous series? Discuss the significance of homologous series.
Ans. A homologous series may be defined as a series or a group of similarly constituted organic compounds having the same functional group and general formula arranged in order of their increasing molecular mass. Any two successive members of a homologous series differ in their molecular formula by a CH2 -group or unit. The members of a homologous series are called homologues.
If the method of preparation and properties of a certain member of a homologous series known, then the method of preparation and properties of the other members of the same series can be easily predicted.
Q.4 Mention the important characteristics of a homologous series.
Ans. (1) All the members of a homologous series have the same constituent elements and they can be represented by a single general formula. (2) If all the members of a homologous series are arranged in increasing order of their molecular masses, then two consecutive members of the family differ from one another by one CH2 – group and the difference in their molecular mass is 14 units. (3) All the members of a homologous series have almost similar chemical properties.
Q.5 How was Berzelius’ ‘vital force theory’ proved invalid?
Ans. In 1828, scientist Wohler accidentally prepared urea, an organic compound, by heating an inorganic compound, ammonium cyanate. Later, in 1845, Kolbe and in 1856, Berthelot prepared acetic acid and methane respectively from their constituent elements in the laboratory. All these discoveries proved that no vital force inherent to living beings was involved in the formation of organic compounds. Thus, the ‘vital force theory’ was proved invalid.
Q.6 Why are organic compounds generally insoluble in water (a polar solvent)?
Ans. As organic compounds are formed by covalent bonds, they do not dissociate or ionise in water or any other polar solvents. Consequently, no electrostatic force of attraction develops between the water molecules and the molecules of the organic compound. So, organic compounds are insoluble in water. However, some organic compounds dissolve in water by forming hydrogen bonds with water molecules (such as, alcohols, glucose etc.) or by ionising in water (such as, carboxylic acids).
Q.7 Why are the solutions of organic compounds generally non-conductor of electricity?
Ans. As organic compounds are formed by covalent bonds, they do not ionise in water or any other polar solvents. Hence, they cannot conduct electricity in solutions. However, aqueous solutions of carboxylic acids such as, formic acid, acetic acid etc., ionise in water and conduct electricity to a small extent.
Q.8 What is catenation property of carbon?
Ans. The unique self-linking property of carbon due to which C-atoms mutually combine with each other by forming covalent single, double or triple bonds to form long open chains (straight or branched) and rings of different sizes is called catenation property of carbon. Due to this reason, carbon forms a vast number of organic compounds.
Q.9 Why is carbon able to show catenation carbon able to show property?
Ans. Due to the small size and moderate electronegativity of carbon atom, it can form very strong and stable carbon-carbon covalent bond. As a result, carbon atoms can link with one another by forming covalent single, double or triple bonds. For these reasons, carbon is able to show catenation property which leads to the formation of a large number of open chain and cyclic compounds.
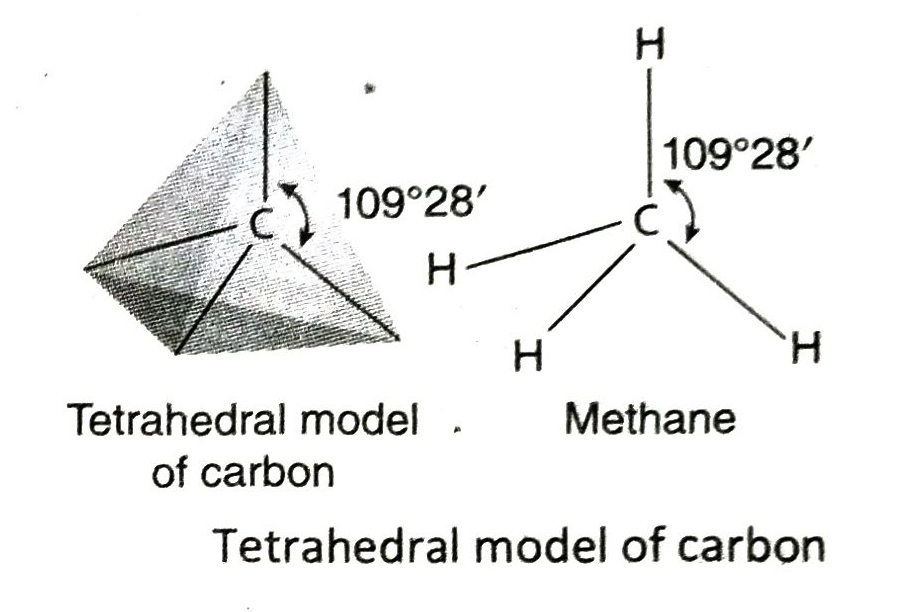
Q.10 Briefly describe the tetrahedral model of the four valencies (bonds) of carbon as proposed by scientists van’t Hoff and Le Bel.
Ans. In 1874, scientists van’t Hoff and Le Bel proposed the tetrahedral model of the four valencies of carbon. According to this model-
- All the four valencies of a carbon atom cannot be on the same plane.
- If a carbon atom is placed at the centre of an imaginary regular tetrahedron, then the four valencies of the C-atom will be directed towards the four corners of the tetrahedron.
- The angle between any two valencies of the carbon atom is 109°28′.
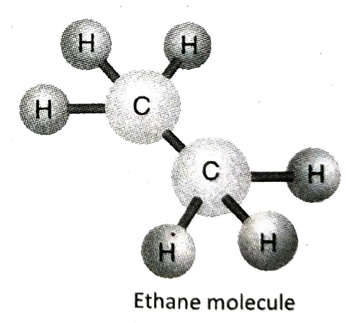
Q.11 Describe the structure of ethane (C2H6) with the help of a suitable diagram.
Ans. In ethane (C2H6), the two adjacent C-atoms equally share one electron pair among themselves to form a single bond. Each of the C-atoms then satisfies its remaining three valencies by forming three single bonds with three hydrogen atoms.
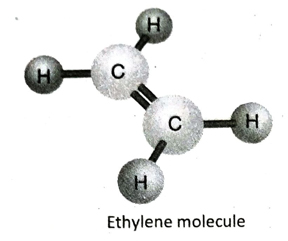
Q.12 Describe the structure of ethylene (C2H4) with the help of a suitable diagram.
Ans. In ethylene (C2H4), the two adjacent C-atoms share two electron pairs with each other to form a double bond and satisfies two of its valencies. Each of the two C-atoms then fulfills its remaining two valencies by forming single bond with two hydrogen atoms each. All the atoms in the molecule remain on the same plane.
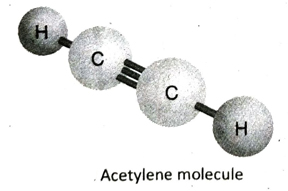
Q.13 Describe the structure of acetylene (C2H4) with the help of a suitable diagram.
Ans. In acetylene (C2H4), the two adjacent C-atoms share three electron pairs with each other to form a triple bond and satisfies three of its valencies. Each of the two C-atoms then fulfills its remaining valency by forming a single bond with one hydrogen atom each. All the atoms in the molecule remain in the same line and so, the molecule is linear in shape..
Q.14 What do you mean by functional group? Give one example.
Ans. An atom or a group of atoms present in an organic compound which determines the nature and characteristic chemical properties of the compound is called a functional group.
Example: —OH is the functional group of the alcohol series.
Q.15 Write the name and structure of the functional group of the alcohol series. Give an example of a compound containing that functional group.
Ans. The functional group of the alcohol series is hydroxyl group and its structure is given by
A compound containing —OH as the functional group is ethyl alcohol (C2H5OH).
Q.16 Write the name and structure of the functional group present in aldehydes. Give an example of a compound containing that functional group.
Ans. The functional group present in ketones is keto group and its structure is given by:
A compound containing —CHO as the functional group is acetaldehyde (CH3CHO).
Q.17 Write the name and structure of the functional group present in carboxylic acids. Give an example of a compound containing that functional group.
Ans. The functional group present in carboxylic acids is carboxyl group and its structure is given by:
A compound containing —COOH as the functional group is acetic acid (CH3COOH).
Q.18 What is functional group isomerism? Give example.
Ans. The phenomenon of existence of two or more compounds having the same molecular formula but different functional groups (i.e., belonging to different families) is called functional group isomerism.
Example: The functional groups in dimethyl ether (CH3—O—CH3) and ethyl alcohol (C2H5OH) are —O— and —OH respectively but both of these compounds have the same molecular formula of C2H6O.
Q.19 What is positional isomerism? Give example.
Ans. The phenomenon of existence of two or more compounds having the same structure of the carbon chain, i.e., the same carbon skeleton, but differing in the position of the multiple bond or functional group is called positional isomerism.
Example: The compounds, propan-1-ol or n-propyl alcohol (CH3CH2CH2OH) and propan-2-ol or isopropyl alcohol [CH3CH(OH)CH3] both have the same molecular formula C2H8O, but the positions of —OH group is different in both the compounds.
Q.20 The members of a homologous series generally show similar chemical properties but different physical properties. Why?
Ans. Due to the presence of the same functional group, the members of a particular homologous series show similar chemical properties. However, as the molecular masses of the members of a homologous series are different, the physical properties (such as, melting point, boiling point, density etc.) dependent on molecular mass of a compound are also different for the homologues.
Q.21 Give the IUPAC names and structural formulas of the alkynes containing upto 3 C-atoms.
Ans. HC ≡ CH: ethyne
H3C — C ≡ CH: propyne
Q.22 Give the IUPAC names and structural formulas of the carbony! compounds containing upto 3 C-atoms.
Ans. Aldehydes: HCHO: methanal
CH3CHO : ethanal
CH3CH2CHO : propanal
Ketones: CH3COCH3 : propanone
Q.23 Give the IUPAC names and structural formulas of the carboxylic acids containing arboxylic acids upto 3 C-atoms.
Ans. HCOOH : methanoic acid
CH3COOH: ethanoic acid
CH3CH2COOH : propanoic acid
Q.24 (i) Who proved that the main component of all organic compounds is carbon?
(ii) All organic compounds contain carbon but all carbon compounds are not organic are not or compounds. Explain
Ans. (i) Scientist Lavoisier proved that the main component of all organic compounds is carbon.
(ii) All carbon compounds are not organic compounds. Compounds like carbon monoxide, carbon dioxide, metallic carbonate, metallic bicarbonate, metallic cyanite etc. can not be termed as organic compounds as catenation property of carbon, isomerism etc, are not seen in these compounds. They all are inorganic compounds.
Q.25 What is saturated compound? Give is saturated examples.
Ans. Organic compounds, in which all of the carbon atoms are attached through covalent single bond are called saturated compounds.
Examples of saturated compounds are-
Q.26 What is unsaturated compound? Give examples.
Ans. Organic compounds, in which at least two carbon atoms are attached through covalent double or triple bond, are called unsaturated compounds.
Examples of unsaturated compounds are-
Q.27 Why is C2H6 termed as saturated hydrocarbon but C2H4 is termed as is termed as unsaturated hydrocarbon?
Ans. All the carbon atoms of C2H6 are attached through covalent single bond, hence it is termed as saturated hydrocarbon.
On the other hand, in C2H4, two adjacent carbon atoms are attached through covalent double bond and hence it is termed as unsaturated hydrocarbon.
Q.28 What do you mean by isomerism and isomer?
Ans. The phenomenon of existence of two or more molecular compounds possessing the same formula but different physical and chemical properties is known as isomerism.
Compounds with same molecular formula but different physical and chemical properties, are termed as isomers to each other.
Q.29 Write down the definition and example of structural isomerism.
Ans. The phenomenon of existence of two or more compounds having the same molecular formula but different structures are called structural isomerism. For example, two structural isomers having the molecular formula C2H6O are–
H3C—CH2—OH (ethanol)
and H3C—O—CH3 (dimethyl ether)
Q.30 How would you identify two isomeric compounds denoted by formula C2H6O?
Or, Distinguish between ethanol and dimethyl ether by a chemical reaction.
Ans. Two compounds having same formula of C2H6O are dimethyl ether and ethanol. Ethanol reacts with sodium metal at room temperature, forming sodium ethoxide and H2 gas. But dimethyl ether does not react with sodium.
2CH3CH2OH + 2Na → 2CH3CH2ONa + H2↑
CH3—O—CH3 +Na → no reaction
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. The inorganic compound from which an organic compound was first synthetically prepared is
A. ammonium phosphate
B. ammonium chloride
C. ammonium cyanate
D. ammonium sulphate
Ans. C
2. The first synthetically prepared organic compound is
A. benzene
B. urea
C. methane
D. lactic acid
3. In mammals, urea is found in their
A. urine
B. blood
C. saliva
D. cells
Ans. A
4. Organic compounds are generally insoluble in
A. benzene
B. chloroform
C. alcohol
D. water
Ans. D
5. Which of the following properties of carbon is responsible for the formation of a large number of organic compounds?
A. catenation property
B. isomerism
C. tendency to form multiple bonds
D. all of these
Ans. D
6. Carbon shows catenation property because
A. carbon forms covalent bonds
B. C-C bond energy is very high
C. electronegativity of carbon is slightly greater than hydrogen
D. carbon shows tetracovalency
Ans. B
7. The structure of methane is
A. planar
B. regular tetrahedral
Ans. B
8. The value of H—C—H bond angle in methane is
A. 105°
B. 110°
C. 109°28′
D. 108°19′
Ans. C
9. Which of the following is a saturated compound?
A. C2H2
B. C2H4
C. C2H6
D. C3H4
Ans. C
10. Which of the following is an unsaturated compound?
A. C3H8
B. C3H4
C. C2H5OH
D. C3H6O
Ans. B
11. The number of covalent bonds in the compound, C4H10 is
A. 13
B. 9
C. 8
D. 12
Ans. A
12. The number of single bonds in the alkyne having molecular formula C4H6 is
A. O
B. 1
C. 8
D. 2
Ans. C
13. Which of the following compounds has a linear structure?
A. C2H6
B. C2H4
C. C2H2
D. all of these
Ans. C
14. In which of the following compounds all of its atoms remain on the same plane though it is not structurally linear?
D. none of these
Ans. B
15. The type of structural isomerism shown by n-butane and isobutane is
A. positional isomerism
B. chain isomerism
C. functional group isomerism
D. metamerism
Ans. B
16. Ethyl alcohol and dimethyl ether are
A. positional isomers
B. chain isomers
C. functional group isomers
D. metamers
Ans. C
17. The type of isomerism shown by n-propanol and isopropanol is
A. positional isomerism
B. chain isomerism
C. functional group isomerism
D. metamerism
Ans. A
18. The difference in molecular mass of two consecutive members in a homologous series is
A. 10
B. 12
C. 14
D. 16
Ans. C
19. The member of the carboxylic acid family with the lowest molecular mass is
A. formic acid
B. acetic acid
C. propionic acid
D. none of these
Ans. A
20. Which of the following is not a saturated hydrocarbon?
A. CH4
B. C2H6
C. C2H4
D. C3H8
Ans. C
21. Which one of the following is also named as paraffin?
A. alkene
B. alkyne
C. alkane
D. none of the above
Ans. A
22. Formula of the simplest alkene is
A. C2H2
B. C2H4
C. C2H6
D. CH4
Ans. B
23. Compound with covalent triple bond
A. ethane
B. acetylene
C. ethelene
D. methane
Ans. B
24. Which of the following pair are isomer to each other?
A. methanol and ethanol
B. dimethyl ether and ethyl alcohol
C. acetaldehyde and acetone
D. formic acid and methanol
Ans. B
Answer in brief
1. Give the chemical equation for the reaction by which urea is produced from ammonium cyanate.
Ans. The chemical equation for the reaction by which urea is produced from ammonium cyanate is-
2. Give some examples of organic solvents.
Ans. Some examples of organic solvents are benzene, chloroform, acetone, ethanol etc.
3. What are hydrocarbons?
Ans. The binary compounds of carbon and hydrogen are called hydrocarbons.
4. What are saturated hydrocarbons?
Ans. The hydrocarbons in which all the C-atoms are linked to one another through single covalent bonds only are called saturated hydrocarbons. For example, methane, ethane, propane.
5. What are unsaturated hydrocarbons?
Ans. The hydrocarbons containing at least a double or a triple bond are called unsaturated hydrocarbons. For example, ethylene, acetylene.
6. Why are alkanes also known as paraffins?
Ans. Alkanes are also known as paraffins due to their less reactive nature.
7. To which class does the organic compounds containing —CHO group belong?
Ans. The organic compounds containing —CHO as the functional group belongs to aldehyde.
8. To which class does the organic compounds containing —CO— as the functional group belongs?
Ans. The organic compounds containing —CO— as the functional group belongs to ketone.
9. To which class does the organic compounds containing —COOH as the functional group belongs?
Ans. The organic compounds containing —COOH as the functional group belongs to carboxylic acid.
10. To which class does the organic compounds containing —NH2 as the functional group belongs?
Ans. The organic compounds containing —NH2 as the functional group belongs to amine.
11. To which class does the organic compounds containing —O— as the functional group belongs?
Ans. The group name for organic compounds containing —O— as the functional group is ether.
12. Name the first member of the alcohol homologous series. What is its IUPAC name?
Ans. The first member of the alcohol homologous series is methyl alcohol (CH3OH).
Its IUPAC name is methanol.
13. Name the first member of the aldehyde homologous series. What is its IUPAC name?
Ans. The first member of the aldehyde homologous series is formaldehyde (HCHO).
Its IUPAC name is methanal.
14. Name the first member of the ketone homologous series. What is its IUPAC name?
Ans. The first member of the ketone homologous series is acetone (CH3COCH3).
Its IUPAC name is propanone.
15. Name the first member of the carboxylic acid homologous series. What is its IUPAC name?
Ans. The first member of the carboxylic acid homologous series is formic acid (HCOOH). Its IUPAC name is methanoic acid.
16. Name the first member of the amine homologous series. What is its IUPAC name?
Ans. The first member of the amine homologous series is methylamine (CH3NH2).
Its IUPAC name is methanamine.
17. Name the first member of the ether homologous series. What is its IUPAC name?
Ans. The first member of the ether homologous series is dimethyl ether (CH3OCH3).
Its IUPAC name is methoxymethane.
18. What is isomerism?
Ans. The phenomenon of existence of two or more compounds having the same molecular formula but different chemical and physical properties is called isomerism.
19. What do you mean by structural isomerism?
Ans. The phenomenon of existence of two or more compounds having the same molecular formula but different physical and chemical properties due to difference in their structures i.e., different bonding sequence is called structural isomerism.
20. Between ethyl alcohol and dimethyl ether, which one does not react with metallic sodium?
Ans. Between ethyl alcohol and dimethyl ether, the latter does not react with metallic sodium.
21. What is the minimum number of C-atoms required to represent the structural formula of a ketone?
Ans. A minimum of three C-atoms are required to represent the structural formula of a ketone.
22. Name the two scientists who proved ‘the vital force theory’ wrong by successfully synthesising acetic acid and methane in the laboratory.
Ans. Kolbe and Berthelot were the scientiests who proved ‘the vital force theory’ to be wrong.
23. Why is ethanol called ‘grain alcohol’?
Ans. Ethanol is also called ‘grain alcohol’ because it can be prepared by the fermentation of finely ground grains (such as corn).
24. Which type of chemical bond is present in organic compounds?
Ans. Covalent bond.
25. Give example of a biomolecule.
Ans. Glucose.
26. Write down the number of H-atoms present in 4C-alkane.
Ans. Number of H-atoms in a 4C-alkane is 10.
27. What is the number of H-atoms in a 3- carbon alkyne?
Ans. Number of ‘H’-atoms in a 3- carbon alkyne is 4.
28. Which type of hydrocarbon is acetylene?
Ans. Acetylene is unsaturated hydrocarbon.
Fill in the blanks
1. Organic compounds are generally insoluble in ………… solvents.
Ans. polar
2. Most organic compounds are combustible due to the presence of ……….. and ……….
Ans. carbon, hydrogen
3. Organic compounds do not …………. when dissolved in suitable solvents.
Ans. ionise
4. A carbon atom forms ………… bond with other atoms.
Ans. covalent
5. In most cases, carbon cannot form ………….. compounds.
Ans. ionic
6. In the tetrahedral model of C-atom, all the four bonds of carbon atom does not remain on the ……….. plane.
Ans. same
7. In the tetrahedral arrangement of four valencies of carbon, the carbon atom is present at at the ……….. of the tetrahedron and the four bonds are directed towards the four …………… of the tetrahedron.
Ans. centre, corners
8. The large number of organic compounds exists due to ………… and ………… of carbon.
Ans. catenation, isomerism
9. The chain formed by carbon atoms due to catenation may be either ………… or ………..
Ans. open, cyclic
10. In methane, all the four valencies of carbon atom are satisfied by …………
Ans. single bonds
11. The …………. of an organic compound determines its chemical property.
Ans. functional group
12. The chemical reactivity of saturated hydrocarbons is very ………… while that of unsaturated hydrocarbons is quite ………..
Ans. less, high
13. Between alcohols and ethers, ……….. are relatively less reactive.
Ans. ethers
14. Between aldehydes and ketones, …………… are chemically more reactive.
Ans. aldehyde
15. If an alcohol and an ether are isomers, then it is an example of …………. isomerism.
Ans. functional group
16. Two adjacent members of a homologous series differ in their molecular formula by a ……… group.
Ans. CH2
17. All the compounds of a homologous series contain the same ………….
Ans. functional group
18. The chemical properties of all the members of a homologous series is generally …………
Ans. identical
19. The phenomenon of similarity in the properties of the members of a homologous series is known as …………
Ans. homology
20. When one hydrogen atom is removed from ethane, ……….. group is formed.
Ans. ethyl
State whether true or false
1. The first organic compound to be synthesised from an inorganic compound is acetic acid.
Ans. False
2. Methane and ethane are saturated hydrocarbons while ethylene and acetylene are unsaturated hydrocarbons.
Ans. True
3. In C2H2, the two adjacent carbon atoms are linked by a double bond.
Ans. False
4. Alkenes are also called olefins because the lower members of this family react with halogens to form oily substances.
Ans. True
5. Ethylene reacts with sulphur monochloride (S2Cl2) to produce mustard gas.
Ans. True
6. CnH2n+1 OH is the general formula of carboxylic acids.
Ans. False
7. 2-bromopentane and 3-bromopentane are positional isomers.
Ans. True
8. The molecular formula, C3H6O, represents two functional isomers namely, acetone and propionaldehyde.
Ans. True
9. Thermal decomposition of organic compounds is known as pyrolysis.
Ans. True
10. The first member of the aldehyde family is formaldehyde and its IUPAC name is ethanal.
Ans. False
11. The IUPAC name of dimethyl ether is methoxymethane.
Ans. True
TOPIC – B
Methane, Ethylene, Acetylene, LPG and CNG
SHORT AND LONG ANSWER TYPE QUESTIONS
Q.1 What are the important uses of methane?
Ans. (1) ‘Carbon black’ obtained due to cracking of methane is used in making black paint, printing ink and in rubber industries for making motor tyres.
(2) Important chemical compounds such as methyl chloride, acetylene, formaldehyde, methanol are prepared from methane. (3) Due to high calorific value of methane (1000 Btu/ft3), it is used as a fuel.
Q.2 Mention some important uses of ethylene.
Ans. (1) Ethylene is used in ripening and preservation of fruits. (2) It is used in the preparation of ethylene dichloride, ethylene dibromide, mustard gas (toxic in nature) etc. (3) It is used in the manufacture of plastics such as polythene, polystyrene etc., and synthetic fibres like nylon, terylene etc. (4) A mixture of 80% ethylene and 20% oxygen is used as an anesthetic in surgeries.
Q.3 Mention some important uses of acetylene.
Ans. (1) Acetylene is used for producing oxy-acetylene flame, used for cutting steel and other metals. (2) It is used to produce bright illuminating flame in carbide lamps. (3) It is used in the industrial preparation of acetaldehyde, acetic acid, ethyl alcohol, acetone etc. (4) Acetylene is also used in the manufacture of industrial non-inflammable solvents like acetylene tetrachloride (westron, C2H2C4) and trichloroethylene (westrosol, Cl2C = CHCl) used in dissolution of fats, oil and resins.
Q.4 Write some important uses of LPG.
Ans. (1) As the calorific value of LPG is very high (29500 kcal/m³), it is used as a fuel for cooking purposes. (2) It is also used as a fuel in vehicles and industries. (3) As boiling point of LPG is quite low, it is also used as a refrigerant.
Q.5 What are the advantages of using LPG as a fuel?
Ans. (1) As the calorific value of LPG is very high, it easily generates large amount of heat which is used up in heating other objects. (2) LPG undergoes complete combustion. So, it does not produce ashes or liberate poisonous gas such as CO during its combustion. (3) Using a regulator, the flow of LPG from the cylinder can be controlled. Hence, its combustion can be controlled depending upon the requirement of heat. (4) LPG cylinders can be conveniently transported to far off places as well. (5) It does not contain carbon monooxide and hence it is not harmful.
Q.6 Which sulphur compound is mixed with LPG? Why is this compound added?
Ans. Ethyl mercaptan (C2H5SH) is mixed with LPG. It emits a very foul smell.
The gases constituting LPG are colourless and odourless but are highly imflammable. So, if these gases accidentally leak out of the cylinder, it is not possible to detect them which may lead to fatal accidents. So, as a precautionary measure, ethyl mercaptan is added to LPG as its characteristic foul smell help to detect any gas leakage thereby preventing accidents.
Q.7 Mention some important uses of CNG. Mention one environmental disadvantage of using CNG.
Ans. (1) As the calorific value of CNG is very high (21300 Btu/lb), it is widely used as a fuel. (2) CNG causes least air pollution when it is used in vehicles. So, nowadays CNG is extensively used as a fuel in public transports like buses, autos, taxis etc.
Small amount of methane is released into the atmosphere due to usage of CNG. Methane being a greenhouse gas causes global warming.
Q.8 What are the advantages of using CNG as a fuel?
Ans. (1) The carbon content of CNG is very low. So, it produces very less amount of carbon particles and CO2 compared to other fuels during its combustion. Thus, CNG causes less air pollution. (2) It has a very high ignition temperature (1350°F). Hence, CNG does not burn easily which makes it safe to use. (3) No poisonous gas and ash are produced during combustion of CNG. (4) The calorific value of CNG (21300 Btu/ lb) is higher than that of diesel and LPG.
Q.9 What are the disadvantages of using CNG as a fuel?
Ans. (1) As it is a gaseous fuel, the amount of heat generated by CNG per unit volume is low compared to other fuels. (2) The tanks required to store CNG are usually 3 to 5 times larger than those required for other fuels like diesel. (3) CNG being lighter than air rises up and rapidly spreads in the atmosphere when it comes out of a cylinder. Consequently, the entire place gets covered by CNG leading to serious explosion. (4) During combustion of CNG, small amount of methane which is a greenhouse gas, is released into the atmosphere.
Q.10 Mention the industrial sources of methane (CH4).
Ans. (1) The natural gas obtained from petroleum mines contains large amount of methane (40-90%). (2) Coal gas contains almost 40% (by volume) methane. (3) Methane is found in traces in the gas obtained from coal mines. (4) A mixture of CO2 and H2 or CO and H2 when passed over hot Ni at 250-400°C, methane is produced.
Q.11 Mention the major industrial sources of ethylene (C2H4).
Ans. (1) The natural gas obtained from petroleum mines contains almost 20% of ethylene. (2) Ethylene is found in large volumes in coke oven gas. (3) Coal gas contains small amount of ethylene (almost 4%). (4) During cracking (the process by which long-chain hydrocarbons are degraded into smaller hydrocarbons by applying heat) of petroleum, ethylene is produced as a by-product.
Q.12 Mention the major industrial sources of acetylene (C2H2).
Ans. (1) Coal gas contains traces of acetylene (almost 0.06%) (2) Acetylene is produced by decomposing natural gas (mainly methane) at high temperature.
Q.13 What is LPG? Mention its industrial on its in source?
Ans. LPG stands for Liquefied Petroleum Gas. It is a mixture of hydrocarbons of low molecular mass (upto 3-4 C-atoms). The major constituents of LPG are n-butane, propane, isobutane, butene etc. It also contains small amounts of propylene and ethane.
LPG is obtained by compressing the crude petroleum during refining into liquid under high pressure. It is stored in steel cylinders.
Q.14 Mention the onstituents and industrial sources of CNG.
Ans. Constituents: The major constituent of CNG (Compressed Natural Gas) is methane (almost 90%). Apart from this, trace amounts of ethane, ethene, propane, butane and low-boiling pentane are also present in CNG.
Industrial sources: Natural gas is obtained above petroleum in petroleum mines and also from the coal mines. This gas is compressed into liquid by applying high pressure.
Q.15 Write down the hydrogenation reaction of ethylene specifying suitable conditions and chemical equation.
Ans. Hydrogen adds to the double bond of ethylene molecule at ordinary temperature and pressure in the presence of finely divided platinum or palladium or Raney nickel or at a temperature of about 200-300°C in the presence of finely divided nickel catalyst to produce ethane.
Q.16 Write a short note on polymerisation of ethylene.
Ans. When ethylene is heated at 150-200°C in the presence of oxygen or peroxide catalyst under a very high pressure (1500-2000 atm), a large number of ethylene molecules combine with each other to form a solid compound of high molecular mass (approx. 20000) called polyethylene or polythene. This reaction is known as polymerisation of ethylene.
Q. 17 Write down the hydrogenation reaction of acetylene specifying the suitable conditions and chemical equation.
Ans. Hydrogen reacts with acetylene at ordinary temperature in presence of Raney nickel or powdered platinum or palladium catalyst or at a temperature of about 200 – 300°C in presence of powdered nickel catalyst to produce ethane. The reaction occurs in two steps. In the first step, one molecule of hydrogen adds to acetylene to form ethylene and in the second step, another molecule of hydrogen adds to ethylene to form ethane.
Q.18 Three cylinders separately contain methane, ethylene and acetylene. How will you identify the gases?
Ans. At first, the three gases are separately passed through ammoniacal cuprous chloride solution. The gas which produces a red precipitate is acetylene. After that, the two remaining gases are separately passed through bromine dissolved in CCl4 solution. The gas which turns orange-brown solution of bromine colourless is ethylene. Thus, the remaining gas is definitely methene.
Q.19 What is Will-o’-the-wisp?
Ans. Methane is formed in marshy lands due to bacterial decomposition of organic matters. Moreover phosphine (PH3) and diphosphorous tetrahydride (P2H4) are also formed as a result of putrefaction of animal bodies. P2H4 radily burns in air. So when the mixture of CH4, PH3 and P2H4 comes in contact with air, P2H4 sets the gas mixture on fire and the heat produced causes methane to burn with a light blue flame. As a result, an intermittent source of light is produced. This is known as ‘Will-o’-the-wisp’.
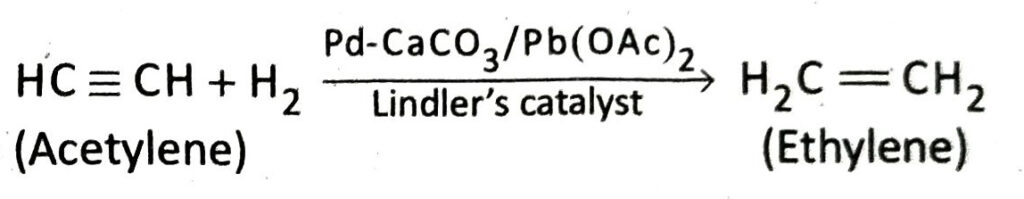
Q.20 How will you prepare ethylene from lene from acetylene?
Ans. In the presence of Lindler’s catalyst, acetylene combines with only one molecule of hydrogen to form ethylene.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. The hydrocarbon from which carbon black is obtained is
A. methane
B. ethane
C. ethylene
D. acetylene
Ans. A
2. The gaseous organic compound used in carbide lamps is
A. methane
B. ethylene
C. acetylene
D. butane
Ans. C
3. Which of the following reagents is used to detect the unsaturation in ethylene?
A. alkaline KMnO4 solution
B. acidic KMnO4 solution
C. neutral KMnO4 solution
D. none of these
Ans. A
4. Which of the following compounds is used for the preservation and ripening of fruits?
A. methane
B. ethane
C. ethylene
D. acetylene
Ans. C
5. Which of the following compounds is used for the preparation of mustard gas?
A. acetylene
B. ethylene
C. ethane
D. methane
Ans. B
6. The major constituent of natural gas obtained from petroleum mines is
A. ethylene
B. acetylene
C. butane
D. methane
Ans. D
7. Methane undergoes stepwise substitution reaction with chlorine in the presence of diffused sunlight. The product formed along with HCl in the second step of this reaction is
A. methyl chloride
B. chloroform
C. carbon tetrachloride
D. methylene chloride
Ans. D
8. At STP, the molar volume of ethylene with respect to that of polythene is
A. very high
B. very less
C. almost equal
D. equal
Ans. A
9. When acetylene gas is passed through reddish yellow coloured bromine water, the latter
A. turns blue
B. turns green
C. turns yellow
D. decolourises
Ans. D
10. Catalyst used to prepare ethane from ethylene at natural temperature is
A. Ni
B. MnO2
C. raney Ni
D. H2SO4
Ans. C
11. Which of the following is formed by addition of water in calcium carbide?
A. C2H2
B. CH4
C. C2H4
D. C2H6
Ans. A
12. In which of the following cases acetylene is used?
A. in industrial preparation of formaldehyde
B. preservation of ripe fruits.
C. preparation of mustard gas
D. préparation of westron
Ans. D
13. Which of the following is used in the preparation of artificial rubber?
A. CH4
B. C2H4
C. C2H2
D. C2H6
Ans. C
Answer in brief
1. What is carbon black?
Ans. At 1000°C, methane decomposes to form fine particles of carbon which is known as carbon black.
2. What are the uses of carbon black?
Ans. Carbon black is used in the manufacture of tyres, typewriter ribbons, printing ink, shoe polish etc.
3. Which member of the alkane homologous series is the major constituent of natural gas?
Ans. Methane (CH4) belonging to the alkane homologous series is the major constituent of natural gas.
4. Give an example of a hydrocarbon which is also a greenhouse gas.
Ans. Methane (CH4) is a hydrocarbon as well as a greenhouse gas.
5. Which hydrocarbon is used in the industrial preparation of formaldehyde?
Ans. Methane (CH4) is used in the industrial preparation of formaldehyde.
6. Which hydrocarbon is used in the manufacture of industrial non-inflammable solvents namely Westron and Westrosol (used to dissolve fats, oils, resins etc.)?
Ans. Acetylene (C2H2) is used in the manufacture of industrial non-inflammable solvents namely Westron and Westrosol.
7. Write with equation what happens when methane undergoes combustion in the presence of sufficient oxygen.
Ans. When methane undergoes combustion it burns with a pale bluish non-luminous flame in presence of sufficient oxygen or air to produce CO2 and water vapour.
CH4 +2O2 → CO2 + 2H2O + heat
(213 kcal mol-1)
8. Name the catalyst used in the addition reaction between ethylene and hydrogen.
Ans. Pt, Pd or Raney nickel is used as the catalyst in the addition reaction between ethylene and hydrogen.
9. Under what conditions do the H-atoms of methane gets replaced by chlorine atoms in stepwise substitution reaction?
Ans. When chlorine reacts with methane in the presence of diffused sunlight the H-atoms of methane gets successively replaced by chlorine atoms in stepwise substitution reaction.
10. What is the main component of marsh gas?
Ans. Main component of Marsh gas is methane (CH4).
11. Write down the formula of the organic compound formed in the first step of the substitutional reaction of methane with chlorine.
Ans. Formula of the compound is CH3Cl (methyl chloride).
12. Which gas is responsible for Will-O’-the wisp?
Ans. Methane gas (CH4) along with phosphine (PH3) and diphosphane (P2H4) are responsible for Will-O’-the wisp.
13. Which one undergoes addition reaction, saturated or unsaturated hydrocarbon?
Ans. Unsaturated hydrocarbon undergoes addition reaction.
14. How will you identify wheather an organic compound is unsaturated or not?
Ans. If the red colour of bromine water decolourises in reaction with the sample organic compound, it can be said that the sample organic compound is unsaturated.
15. What is the main component of LPG?
Ans. Butane (C4H10).
16. Mention an use of LPG.
Ans. LPG is used as fuel in cooking and industrial purpose.
17 Which bad smelling component is mixed with LPG?
Ans. Ethyl mercaptan (C2H5SH)
18. What is the industrial source of CNG?
Ans. The source is usually shale rock far beneath the earth’s surfaces. Again the trapped gas in petroleum mine is CNG.
19. Which one of petrol and CNG causes comparatively lower air pollution when used as fuel for vehicles?
Ans. CNG causes comparatively lower pollution.
20. Mention an use of CNG.
Ans. CNG is used as fuel in bus, taxi, auto rickshaw etc.
Fill in the blanks
1. The characteristic odour of LPG is due to the presence of …………..
Ans. ethyl mercaptan
2. Methane gas is used as a fuel because of its high …………
Ans. calorific value
3. At high temperatures, natural gas decomposed to produce …………..
Ans. acetylene
4. …………. is formed when all the hydrogen atoms in methane are replaced by chlorine atoms.
Ans. Carbon tetrachloride
5. The number of organic compounds formed in the reaction between methane and chlorine is …………
Ans. 4
6. The temperature of oxy-acetylene flame is ………….. °C.
Ans. 3000
7. The major constituent of LPG is …………..
Ans. butane
8. To reduce air pollution, ………… is used as an alternative fuel nowadays in buses, taxis, auto-rickshaws and other vehicles.
Ans. CNG
9. In the presence of excess oxygen, methane burns with a non-luminous, pale ………… flame.
Ans. blue
10. In addition reaction with hydrogen, each molecule of, ethylene combines with ………… molecule of hydrogen.
Ans. one
11. At normal temperature, ethylene is a …………… while polythene is a …………
Ans. gas, solid
12. The component of Marsh gas which ignites in contact with air is ………..
Ans. P2H4
13. 3’H’ atoms of methane when substituted by 3’Cl’ atoms, ……….. is formed.
Ans. Chloroform
14. Full form of LPG is …………..
Ans. liquefied petroleum gas
15. Full form of CNG is …………
Ans. compressed natural gas
State whether true or false
1. Methane usually participates in addition reactions.
Ans. False
2. A mixture of methane and oxygen explodes when it comes in contact with fire.
Ans. True
3. Decomposition of acetylene at a temperature of about 1000 °C produces fine particles of carbon called carbon black.
Ans. False
4. LPG stands for Liquid Petroleum Gas.
Ans. False
5. Acetylene is used to produce illuminating flame in carbide lamps.
Ans. True
6. 10% methane is present in coal gas (by volume).
Ans. False
7. Smell of H2S can be smelled on leakage of LPG cyllinder.
Ans. False
8. CNG produces pollutants than LPG. comparatively lower
Ans. True
TOPIC – C
Polymer, Ethyl Alcohol, Acetic Acid and Denatured Spirit
SHORT AND LONG ANSWER TYPE QUESTIONS
1. Which compound is formed due to polymerisation of ethylene? How does it differ from ethylene in terms of it’s properties?
Ans. Due to polymerisation of ethylene, the polymer polythene or polyethylene is formed.
The difference in properties of ethylene and polythene are as follows-
| Ethylene |
Polythene |
| 1. Ethylene is a gas at ordinary temperature. |
1. Polythene is a solid at ordinary temperature. |
| 2. The molar mass of ethylene is constant. Its value is 28. |
2. Polythene is formed by the combination of numerous ethylene molecules. Thus, its molar mass is very high (approx. 20000). |
| 3. Being a gas, the molar volume of ethylene at STP is very high (22.4 L). |
3. Being a solid, the molar volume of polythene at STP is very low. |
Q.2 What are biodegradable and nonbiodegradable polymers? Give examples.
Ans. The polymers which are degraded by the microenzymatic action of environmental organisms (such as, bacteria, fungi etc.) to form simple molecules (such as CO2, H2O etc.) are known as biodegradable polymers.
Example: Polymers obtained from animals and plants such as carbohydrates (cellulose, starch), protein, nucleic acids etc.
The polymers which are not degraded by the enzymatic action of environmental microorganisms (such as, bacteria, fungi etc.) to form simple molecules such as CO2, H2O etc., are known as non-biodegradable polymers.
Example: Synthetic polymers such as polythene, PVC, polystyrene, teflon etc.
Q. 3 Biodegradable polymers do not cause environmental pollution-Explain with suitable example.
Ans. Biodegradable polymers are degraded into simpler compounds such as CO2, H2O etc., due to the enzymatic action of different microorganisms (bacteria, fungi etc.) present in the environment. These compounds are not hazardous to the environment and thus, biodegradable polymers do not cause environmental pollution.
For example, cotton, straw, paper and wood are biopolymers or polymers originating from plant sources. Cellulose present in these biopolymers are degraded by the enzymatic action of microorganisms into compounds which are not harmful to the environment.
Q. 4 How do non-biodegradable polymers polymers cause environmental pollution?
Ans. (1) Most synthetic polymers are nonbiodegradable in nature and hence, they are not easily decomposed. These polymers accumulate in soil and prevent free flow of air and water in the soil. This makes the soil infertile and unsuitable for agriculture. Deposition of these polymers in the soil leads to the formation of toxic chemical compounds due to weathering which in turn adversely affects the useful soil microorganisms. Accumulation of plastics also blocks the drains and sewage canals which make the sewage system ineffective. (2) Combustion of synthetic polymers produces poisonous gases like CO, SO2, NO2 etc., and causes air pollution. (3) PVC polymers may sometimes contain free vinyl chloride monomer. When water pipes, water tanks etc., made of PVC get damaged due to friction, the monomer (vinyl chloride) may mix with water. Vinyl chloride is carcinogenic in nature.
Q.5 What measures should be adopted to prevent pollution caused by non-biodegradable polymers?
Ans. (1) Extensive use of synthetic polymers such as plastics, polythene, PVC etc., should be reduced. (2) Recently, it has been possible to prepare some synthetic biodegradable polymers such as Nylon2, Nylon-6, PHBV etc. However, the cost of production of these polymers is very high. (3) Natural polymers such as paper, cotton, jute etc., are biodegradable in nature. Thus, use of jute and paper for packaging purpose should be encouraged. The government must also initiate awareness campaigns for people to encourage the use natural polymers. (4) Remoulding of the plastic wastes into other useful substances by recycling is another way to control pollution caused by non-biodegradable polymers.
Q.6 Discuss some important uses of ethyl alcohol.
Ans. (1) Ethyl alcohol is used as a solvent for resins, soaps, varnishes, rayons, scents, pigments, synthetic rubbers, synthetic fibres, medicines, etc. (2) It is used in the preparation of ether, ethyl esters, ethyl halides, chloroform, acetic acid, ethylene, methylated spirit etc. (3) Power alcohol which is used as an automobile fuel is prepared by mixing ethyl alcohol with petrol. (4) In cold countries, a mixture of water and ethanol is used as the anti-freezing agent in radiators of motor vehicles.
Q.7 Mention some important uses of acetic acid.
Ans. (1) Glacial acetic acid (anhydrous) is used as a solvent for many organic compounds. (2) Acetic acid is used to prepare chemical compounds such as, acetone, ethyl acetate, acetic anhydride, acetyl chloride etc. (3) Cellulose acetate, used for the preparation photographic films and synthetic fibres (rayon), is prepared from acetic acid. (4) Vinegar (5-8% aqueous solution of acetic acid) is used as a preservative for fish, meat, etc., and also for making pickles and chutneys.
Q.8 What are polymers and monomers? Give example.
Ans. The giant molecules of high molecular mass formed by the polymerisation of a large number of small molecules, linked together in long chains of varying lengths are called polymers. The small molecules forming the repeating units in polymers are called monomers.
Example: A large number of ethylene molecules combine with each other to form polyethylene. Hence, polyethylene is the polymer while ethylene is its monomer.
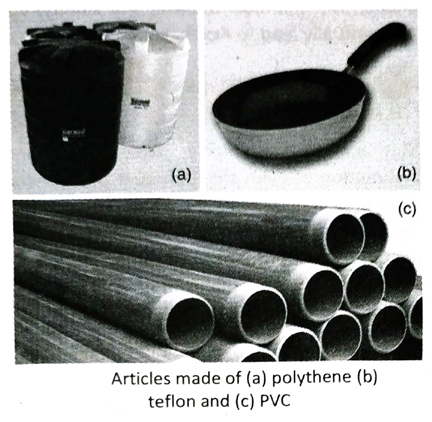
Q. 9 Write the name and structural formula of the monomer of polythene. Write some uses of polythene.
Ans. The monomer of polythene is ethene or ethylene and its structural formula is CH2 = CH2.
Uses of polythene: It is used as a packaging material and in the manufacture of carry bags, water pipes, water tanks, bottles, buckets, window-nets and as electrical insulation for being a non-conductor of electricity.
Q.10 Write the name and structural formula of the monomer of polyvinyl chloride (PVC). Write some uses of PVC.
Ans. The monomer of polyvinyl chloride (PVC) is vinyl chloride and its structural formula is CH2 =CHCl.
Uses of PVC: It is used in the manufacture of electrical wires and cable insulation, water pipes, water tanks, raincoats, artificial flooring, hand bags, tubings and hoses for corrosive materials etc.
Q.11 Write the name and structural formula of the monomer of polytetrafluoroethylene (PTFE) or teflon. Write some uses of teflon.
Ans. The monomer of teflon is tetrafluoroethylene and its structural formula is F2C = CF2.
Uses of teflon: It is used in making non-stick cookwares, as insulating cover in electrical appliances, in making pipes and tanks for carrying corrosive substances and for making laboratory apparatus.
Q.12 Biodegradable polymers are more ecofriendly than non-biodegradable polymers. Explain.
Ans. Uncontrolled use of non-biodegradable polymers is a major cause of environmental pollution. So, use of natural polymers such as cotton, wood, paper, jute etc., should be encoumanufacture of raged until and unless the cost biodegradable polymers can be minimised. Jute and paper should be extensively used for packaging. Jute bags instead of plastic bags should be used for carrying things. As these are biodegradable polymers, they do not cause pollution.
Q.13 Write with equation what happens when absolute ethanol is heated with excess concentrated H2SO4 at 170°C. What will happen if the reaction is carried out with excess ethanol instead of excess acid?
Ans. When absolute ethanol is heated with excess concentrated H2SO4 at 170°C, ethanol undergoes dehydration to produce ethene.
If the reaction is carried out in presence of excess ethanol instead of excess acid at a temperature of 140°C, then diethyl ether is produced instead of ethene.
Q.14 Write with equation what happens when sodium hydroxide reacts with acetic acid.
Ans. Acetic acid reacts with sodium hydroxide solution to form sodium acetate or sodium ethanoate (CH3COONa) and water.
CH3COOH + NaOH → CH3COONa + H2O
Q.15 Write with equation what happens when sodium bicarbonate reacts with acetic acid.
Ans. Acetic acid (CH3COOH) reacts with sodium bicarbonate (NaHCO3) to form sodium acetate, water and carbon dioxide and the latter comes out of the reaction mixture as effervescence.
Q.16 How will you distinguish between ethanol and acetic acid using sodium bicarbonate?
Ans. Ethanol does not react with sodium bicarbonate but, acetic acid reacts with sodium bicarbonate to form carbon dioxide which comes out of the solution as effervescence.
Q.17 What is esterification? Describe the esterification reaction of ethyl alcohol and acetic acid along with suitable equation.
Ans. In presence of a suitable catalyst such as concentrated H2SO4 or dry HCl, carboxylic acids react with dry alcohols to produce esters and water. This reaction is known as esterification reaction.
When dry ethyl alcohol (CH3CH2OH) is heated with acetic acid (CH3COOH) in presence of concentrated H2SO4 which acts as the catalyst, ethyl acetate (CH3COOCH2CH3) having a fruity smell is formed.
Q.18 What are the physiological effects of consuming ethyl alcohol?
Ans. Consumption of limited amount of ethyl alcohol may act as a mild stimulant. However, if it is consumed in large quantities, it may lead to different physiological problems such as, headache, nausea, reluctance to work, incoherence of speech, unconsciousness etc. Regular consumption of ethyl alcohol may lead to addiction which affects the liver and kidneys and may eventually lead to death.
Q.19 Briefly discuss the toxic effects of methanol.
Ans. Methanol is a highly toxic compound. Consumption of even small amount of methanol may be fatal. It gets oxidised to form formaldehyde in the liver cells which rapidly reacts with certain components responsible for formation of the cells. As a result, the protoplasm of the cell gets coagulated. Apart from this, methanol also damages the optic nerves which may cause blindness. Excess intake of methyl alcohol may even cause death.
Q.20 What is methylated spirit or denatured spirit? Write its uses.
Ans. In order to prevent the use of ethanol as a beverage, it is made unfit for consumption by adding highly poisonous methyl alcohol (upto 10%) alongwith small amounts of certain compounds having bitter taste such as, pyridine, copper sulphate, naphtha etc. This mixture is known as methylated spirit or denatured spirit.
Uses: It is used as a solvent for paints and varnishes, as a fuel and in lighting stoves.
VERY SHORT ANSWER TYPE QUESTIONS
Choose the correct answer
1. The polymer which is extensively used for the manufacture of non-stick utensils is
A. polyvinyl chloride
B. polythene
C. teflon
D. polystyrene
Ans. C
2. Which of the following is an example of biodegradable polymer?
A. polythene
B. PVC
C. polystyrene
D. cellulose
Ans. D
3. Which of the following is an example of non-biodegradable polymer?
A. starch
B. protein
C. polythene
D. nucleic acid
Ans. C
4. Which of the following is not an example of a biopolymer?
A. starch
B. cellulose
C. protein
D. PVC
Ans. D
5. The major constituent of rectified spirit is
A. methyl alcohol
B. ethyl alcohol
C. formaldehyde
D. ethanal
Ans. B
6. The major constituent of alcoholic drinks is
A. ethanal
B. ethanol
C. propanone
D. propanal
Ans. B
7. The organic compound present in vinegar is
A. formic acid
B. formaldehyde
C. acetic acid
D. acetaldehyde
Ans. C
8. The organic compound used in the preparation of ‘white lead’ paint is
A. ethyl alcohol
B. acetic acid
C. methyl alcohol
D. formaldehyde
Ans. B
9. Cellulose acetate is not used to make
A. photographic plates
B. synthetic fibres
C. cigarette filters
D. synthetic colours
Ans. D
10. Polymer of ethene is
A. tefflon
B. ethylene
C. polythene
D. polystyrene
Ans. C
11. Monomer of PVC is
A. ethylene
B. acetylene
C. vinyl chloride
D. H2O
Ans. C
12. Which one is formed in the reaction of acetic acid and ethanol?
A. acetone
B. adldehyde
C. propanol
D. ester
Ans. D
13. Main component of denatured spirit
A. methanol
B. ethanol
C. pyridine
D. naptha
Ans. B
14. Other name of denatured spirit
A. ethanol
B. rectified spirit
C. methylated spirit
D. acetic acid
Ans. C
15. Amount of ethanol in rectified spirit is
A. 80%
B. 60%
C. 95.6%
D. 20%
Ans. C
16. H2 gas is formed when Na is added to an organic compound of molecular formula C2H6O. The compound will be
A. vinegar
B. ether
C. acetone
D. ethanol
Ans. D
17. Which of the following is not mixed with ethanol is denatured spirit?
A. CH3OH
B. Pyridine
C. CuSO4
D. propanol
Ans. D
18. Which of the following is used as solvent of paint and varnish?
A. methylated spirit
B. rectified spirit
C. power alcohol
D. none of the above
Ans. A
Answer in brief
1. Which polymer is present in plant fibres such as cotton, jute etc.?
Ans. Plant fibres such as cotton, jute etc., are made of the polymer, cellulose.
2. What is biopol?
Ans. The trade name for polyhydroxybutyrate (PHB) is biopol. It is an eco-friendly and biodegradable synthetic polymer.
3. Give an example of a biodegradable synthetic polymer.
Ans. Polyhydroxybutyrate is a biodegradable synthetic polymer.
4. What are the uses of biopol?
Ans. Biopol is widely used to make the single-use products such as disposable cups, shaving razors, surgical threads etc.
5. What is rectified spirit?
Ans. A solution of 95.6% ethanol and 4.4% water is known as rectified spirit.
6. Give an example of an organic compound which turns blue litmus red.
Ans. Acetic acid turns blue litmus red.
7. Which organic compound is used to make pickles and chutneys?
Ans. Vinegar (5-8% aqueous solution of acetic acid) is used to make pickles and chutneys,
8. What is vinegar?
Ans. A 5-8% aqueous solution of acetic acid is commonly known as vinegar.
9. How do biopolymers decompose in natural environment?
Ans. Biopolymers decompose into simple molecules (like CO2, H2O etc.) by the action of different microorganisms (fungi, bacteria etc.) present in the natural environment.
10. Write down the name of the monomer of polythene.
Ans. Monomer of polythene is ethene or ethylene.
11. What is PVC?
Ans. PVC or polyvinyl chloride is the polymer of vinyl chloride (CH2 = CH—Cl).
12. Mention an use of polyvinyl chloride.
Ans. PVC is used to prepare corrugated roofing material.
13. Name a polymer which is used to prepare raincoat, sandal or gumboots.
Ans. PVC or polyvinyl chloride.
14. Write down the full form of PTFE.
Ans. Polytetrafluoroethylene.
15. Mention an use of polytetrafluoroethylene.
Ans. Polytetrafluoroethylene is used to prepare non-stick cooking utensils.
16. Name two natural polymer.
Ans. Cellulose and protein.
17. Which type of polymer is protein?
Ans. Protein is a biodegradable natural polymer.
18. Name the monomer of protein.
Ans. Monomer of protein is amino acid.
19. Mention an use of ethyl alcohol.
Ans. Ethyl alcohol is used to prepare rectified spirit (96.5% ethanol and 4.4% H2O) which is used as antiseptic.
20. What is formed when ethyl alcohol is dehydrated by conc. H2SO4?
Ans. Ethylene is formed when ethyl alcohol is dehydrated by conc. H2SO4.
21. Write down the formula of a compound which can form ester in reaction with ethanol?
Ans. Acetic acid (CH3COOH)
22. Which gas is evolved when NaHCO3 in added to acetic acid?
Ans. Carbon dioxide (CO2)
23. Which compounds are formed in the reaction of CH3COOH with NaOH ?
Ans. Sodium acetate (CH3COONa) and water (H2O).
24. What is glacial acetic acid?
Ans. Acetic acid that contains a very low amount of water (less than 1%) is called anydrous acetic acid or glacial acetic acid. The reason it is called glacial is because it solidifies into white solid acetic acid crystals at 16.7°C,
Fill in the blanks
1. Ethyl alcohol reacts with metallic sodium at ………… temperature to liberate ………….. gas.
Ans. ordinary, hydrogen
2. The monomer of teflon is ………….
Ans. tetrafluoroe-thylene
3. ………… is mixed with petrol to produce power alcohol which is used as an automobile fuel.
Ans. Ethanol
4. Ethyl alcohol on dehydration produces ………….
Ans. ethylene
5. A mixture of 80% ……………. and 20% …………. is used as an anesthetic during surgeries.
Ans. ethylene, oxygen
6. Among all alcohols, …………. is toxic in nature.
Ans. methanol
7. lodine dissolved in …………… is known as tincture of iodine.
Ans. ethyl alcohol
8. The monomer of PVC is …………..
Ans. vinyl chloride
9. ………… is used to prepare gramophone record.
Ans. PVC
10. The polymer of phenol and formaldehyde is ……………
Ans. bakelite
11. Ethyl alcohol raects with metallic sodium at ………….. temperature to form ………… gas.
Ans. normal, hydrogen
12. ………….. is mixed with petrol to form the fuel for motor car called power alcohol.
Ans. Ethanol
13. Almost …………… ethyl alcohol is present in rectified spirit.
Ans. 95.6%
14. ……………. can cause blindness by causing harm to the optic nerve.
Ans. Methanol
State whether true or false
1. Oxygen or peroxide is used as the catalyst during polymerisation of ethylene.
Ans. True
2. The monomer of the polymer teflon is vinyl chloride.
Ans. False
3. Methanol on entering the body can damage the optic nerves.
Ans. True
4. Polystyrene and polythene are examples of non-biodegradable polymers.
Ans. True
5. Petrol mixed with alcohol along with a cosolvent like benzene is called power alcohol.
Ans. True
6. The basicity of acetic acid is 2.
Ans. False
7. In spite of being an organic compound, ethyl alcohol is soluble in water due to its formation of hydrogen bond with water molecules.
Ans. True
8. The reaction between an alcohol and an aldehyde to form an ester in presence of concentrated sulphuric acid is called esterification reaction.
Ans. False
9. Teflon is used to prepare non-stick frying pans.
Ans. True
10. Ethanol causes harm to liver.
Ans. True
11. Denatured spirit is used as solvent of organic substance.
Ans. True
12. Methylated spirit is poisonous due to the presence of methanol.
Ans. True